Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Spondyloarthritis (SpA) is a disease of the working-age individual, with diverse economic and societal implications, including decreased employment rates. Our aim was to investigate the hierarchy of determinants contributing to work impairment in SpA.
Methods: Data was retrieved from the Assessment of SpondyloArthritis international Society (ASAS)-Health Index (HI) international validation study, a cross-sectional international observational study with a longitudinal component for reliability and responsiveness. Patients >60y reporting to be retired were excluded from the analyses (according to usual retirement ages across the 23 participating countries: www.oecd-ilibrary.org). The Work Productivity and Activity Impairment (WPAI) questionnaire was used to assess work productivity loss outcomes: absenteeism, presenteeism, overall work impairment, overall activity impairment. Initial univariable analyses using generalised estimated equations (GEE) explored the association between work-related outcomes (dependent variables) and sociodemographic and clinical variables (independent variables). A manual forward stepwise variable selection procedure was used for the selection of best-fit multivariable models. To avoid collinearity, separate models were built using ASDAS and BASDAI+CRP as independent variables. Lastly, a decision tree was built using Chi-square Automatic Interaction Detector (CHAID), a method of unbiased hierarchical multivariable analysis.
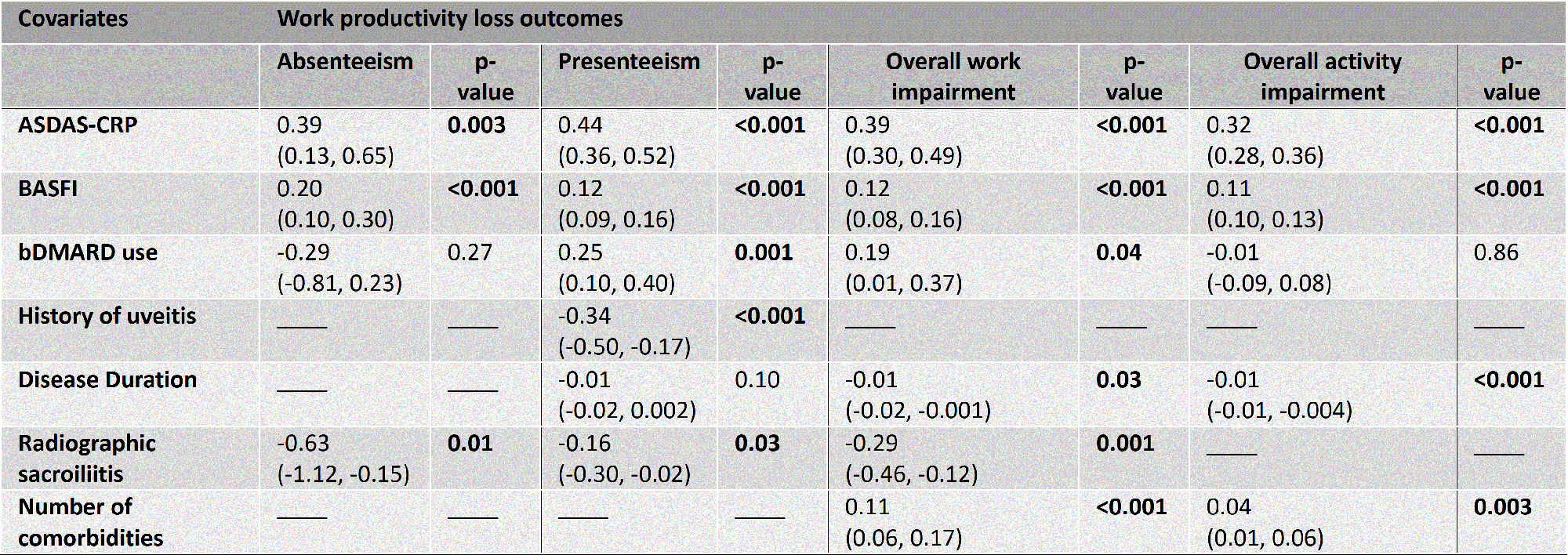
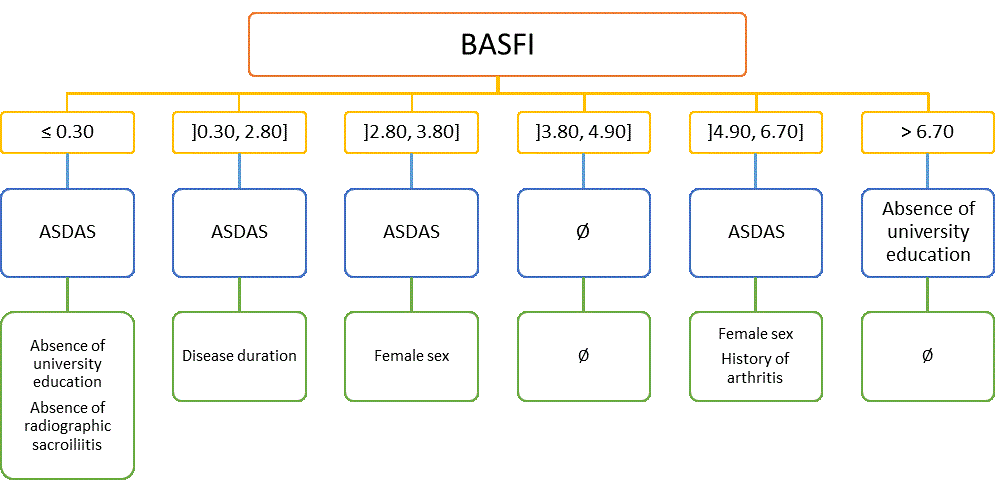
Results: From the original 1548 patients, a total of 1450 patients were included in the analysis, 345 of which had a follow-up reliability/responsiveness visit. Most patients were male (65%), mean age was 40 (±12) years and mean disease duration was 13 (±10) years. Most patients had axial SpA (84%) and a minority had peripheral SpA (16%). Medication use was: NSAIDs 64%, csDMARDs 26% and bDMARDs 38%. Most patients worked full-time (57.1%). Levels of absenteeism were 16% (±32%), presenteeism 29% (±26%), overall work impairment 39% (±34%), and overall activity impairment 41% (±29%). Worse physical function (measured by BASFI) and higher disease activity (measured by ASDAS or by BASDAI+CRP) were independently and consistently associated with all work productivity loss outcomes (Tables). Other variables less consistently associated with work productivity loss outcomes were bDMARD and NSAID use, history of uveitis and peripheral arthritis, disease duration, presence of radiographic sacroiliitis, and level of education (Tables). In the CHAID analysis (Figure), BASFI was the variable with higher discriminative power in predicting overall work impairment; ASDAS was the second-level discriminative variable. University education, disease duration, sex, radiographic sacroiliitis and history of arthritis were the third-level parameters. Similar results were observed for other work productivity loss outcomes (data not shown).
Conclusion: Loss of physical function and higher disease activity are major contributors to work productivity loss and are hierarchically superior to the contribution provided by other demographic and clinical variables.
To cite this abstract in AMA style:
Rajão Martins F, Carvalho P, van der Heijde D, de Hooge M, Boonen A, Braun J, Kiltz U, Machado P. Hierarchy of Determinants of Work Impairment in Spondyloarthritis: Data from the Assessment of Spondyloarthritis International Society Health Index (ASAS-HI) International Validation Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/hierarchy-of-determinants-of-work-impairment-in-spondyloarthritis-data-from-the-assessment-of-spondyloarthritis-international-society-health-index-asas-hi-international-validation-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/hierarchy-of-determinants-of-work-impairment-in-spondyloarthritis-data-from-the-assessment-of-spondyloarthritis-international-society-health-index-asas-hi-international-validation-study/