Session Information
Date: Monday, November 9, 2015
Title: Rheumatoid Arthritis - Clinical Aspects II: Infection, Malignancy and Other Comorbidites in RA
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose:
Tofacitinib
is an oral Janus kinase inhibitor for the treatment of RA. The risk of herpes
zoster (HZ) was elevated within the tofacitinib clinical development program,
although it is unclear how HZ changed over time or whether HZ predicts the
development of other events associated with immunosuppression, such as serious
infection events (SIEs) or malignancy.
Methods: HZ
cases were identified from Phase 1, 2, 3, and long-term
extension (LTE) studies of the tofacitinib RA clinical development program
(data cut-off April 2014; 1 LTE ongoing, database not locked). Crude incidence
rates (IRs; patients with events per 100 patient-years, with 95% confidence
intervals [CIs]) were calculated for HZ during discrete 6-month intervals
of tofacitinib exposure. Characteristics of HZ events were summarized
descriptively. We then evaluated whether HZ predicted subsequent SIE or
malignancy (excluding non-melanoma skin cancer [NMSC]). Crude IRs for these
outcomes were calculated among patients (pts) with/without prior HZ cases. For
SIEs, Cox hazard models were used to evaluate the SIE risk difference between pts
with and without prior HZ cases while controlling for other potential risk
factors. This approach was not possible for malignancies due to the small
number of events.
Results:
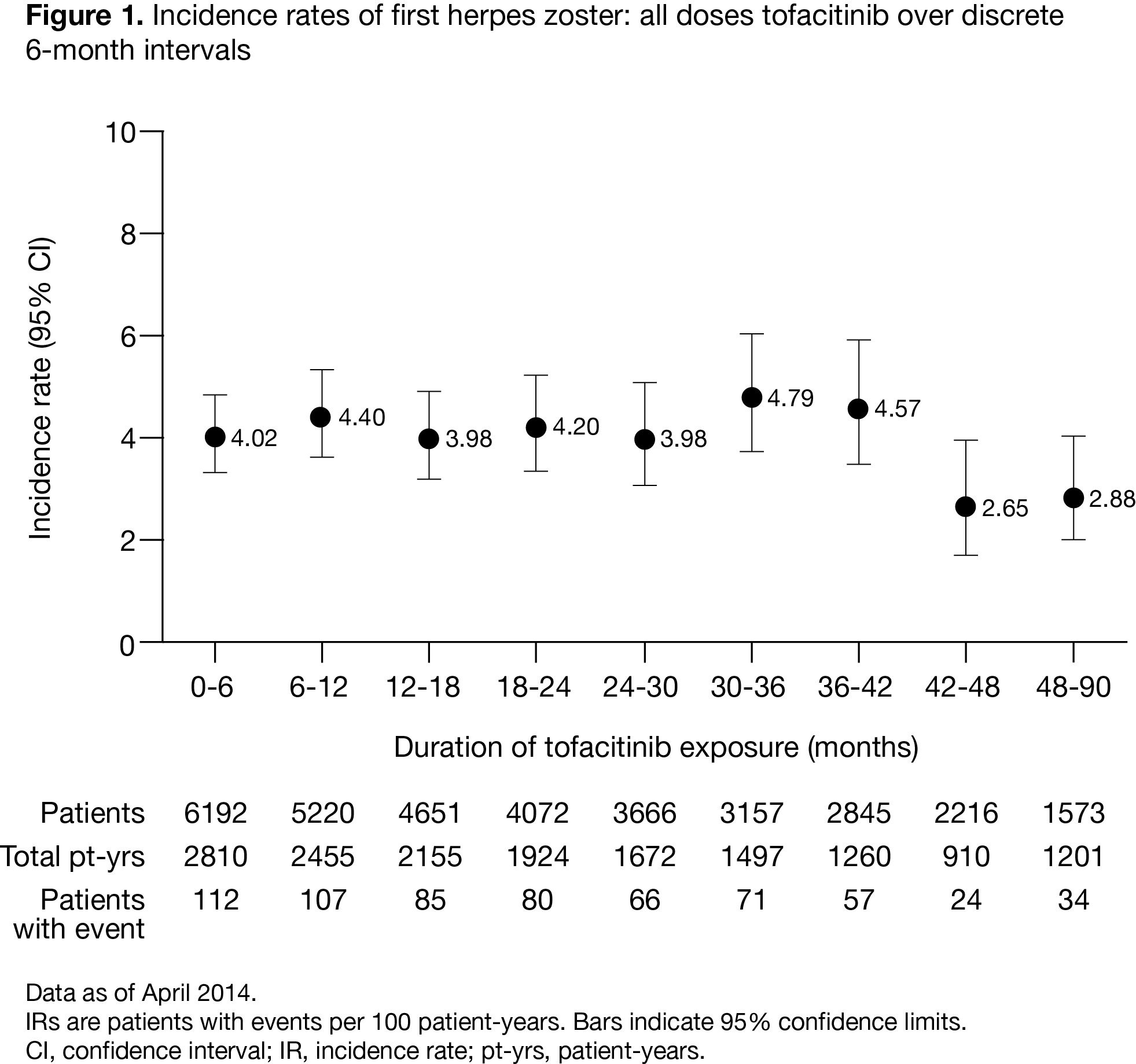
There were 636 tofacitinib-associated HZ
events in 6192 pts with 16,839 patient years of tofacitinib
exposure. The overall IR (95% CI) for HZ was 4.04 (3.73, 4.37), with similar IRs
across exposure durations (Fig 1). Among HZ cases, 84.3% (536) were female,
median age 57 years, and 54.4% (346) were using corticosteroids at baseline. During
tofacitinib exposure, 89.6% (570) of pts with HZ received anti-viral agents,
and 7.4% (47) had a recurrence of HZ. Most HZ first events (93.9%) involved a
single dermatome (95% resolved with treatment; 5% ongoing). None involved
visceral dissemination or death. Post-herpetic neuralgia was infrequent (7.4%).
Discontinuation due to a first HZ event occurred in 8.0% of cases. Among first
non-serious HZ cases, 32 pts had a subsequent SIE (IR: 2.94 [2.01, 4.15])
and 9 pts had a subsequent malignancy (excluding NMSC; IR: 0.81 [0.37,
1.54]). In pts without HZ, IRs for SIEs (2.66 [2.40, 2.94]) and malignancy (0.94
[0.79, 1.12]) were similar. In the multivariable analysis (Fig 2), pts
with HZ cases were no more likely to develop SIEs than pts without prior HZ (Hazard
Ratio 0.77, p=0.172).
Conclusion:
Within
the RA clinical development program for tofacitinib, HZ incidence was stable
over 90 months of exposure. Pts who developed HZ were no more likely to develop
a subsequent SIE or malignancy as compared with those without HZ.
To cite this abstract in AMA style:
Winthrop KL, Tanaka Y, Yamaoka K, Curtis JR, Nduaka C, Fan H, Biswas P, Hirose T, Krishnaswami S, Valdez H, Toyoizumi S, Soma K, Chen C. Herpes Zoster during the Tofacitinib Clinical Development Program for RA: Characterization of Herpes Zoster Incidence and Evaluation of Whether Herpes Zoster Predicts Subsequent Serious Infections or Malignancy [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/herpes-zoster-during-the-tofacitinib-clinical-development-program-for-ra-characterization-of-herpes-zoster-incidence-and-evaluation-of-whether-herpes-zoster-predicts-subsequent-serious-infections-or/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/herpes-zoster-during-the-tofacitinib-clinical-development-program-for-ra-characterization-of-herpes-zoster-incidence-and-evaluation-of-whether-herpes-zoster-predicts-subsequent-serious-infections-or/