Session Information
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: Hepatitis B virus (HBV) reactivation can complicate treatment with immunosuppressive medications. Reactivation risk varies by the status of HBV infection but has been reported in up to one third of patients receiving immunosuppression. While HBV screening is indicated prior to initiation of tumor necrosis factor inhibitors, recommendations are inconsistent regarding HBV screening when prescribing tocilizumab (TCZ) or tofacitinib (TOF), and scant data exist regarding real-world practice. Our objective was to determine providers’ HBV screening practices when prescribing TCZ and TOF.
Methods: We identified patients initiating TCZ or TOF in the Mass General Brigham (MGB) Healthcare System from retrospective review of electronic health records. Adult patients were included if they had ≥ 1 prescription for either TCZ or TOF and an outpatient rheumatology encounter in MGB. We characterized all available HBV screening data (1995-2018) prior to or within 30 days of the first TCZ or TOF prescription as: complete (HBV surface antigen [HBsAg], HBV total core antibody [total anti-HBcAb], and HBV surface antibody [HBsAb]), partial (HBsAg or total anti-HBcAb but not both), and inappropriate (HBV e-antigen, HBcAb IgM, or HBV deoxyribonucleic acid [DNA] without a positive HBsAg or total anti-HBcAb).
Results: Among 913 patients prescribed TCZ and 630 prescribed TOF, we identified 764 patients prescribed TCZ and 495 prescribed TOF who had an outpatient rheumatology encounter in MGB. Median age was 64 years and 60 years in the TCZ and TOF groups, respectively; most were white and female. Rheumatoid arthritis was the most common indication (TCZ [51%] and TOF [77%]) (Table 1). Complete HBV screening was performed in 212 (28%) TCZ patients and 108 (22%) TOF patients (Table 2 and Figure 1). Partial HBV screening was performed in 278 (36%) TCZ patients and 189 (38%) TOF patients. Inappropriate HBV testing was performed in 163 (22%) TCZ patients and 177 (36%) TOF patients. No appropriate HBV screening was performed in 210 (27%) TCZ patients and 158 (32%) TOF patients.
Conclusion: Fewer than 30% of patients prescribed TCZ or TOF for rheumatologic indications had complete HBV screening, which underscores the lack of consistent guidelines regarding HBV screening for this population. More than a quarter had inappropriate HBV screening, which contributes to medical errors and healthcare resource waste. The frequency of partial, inappropriate, or absent HBV screening among patients prescribed TCZ or TOF suggests that provider education and electronic health record system alerts could improve HBV screening practices.
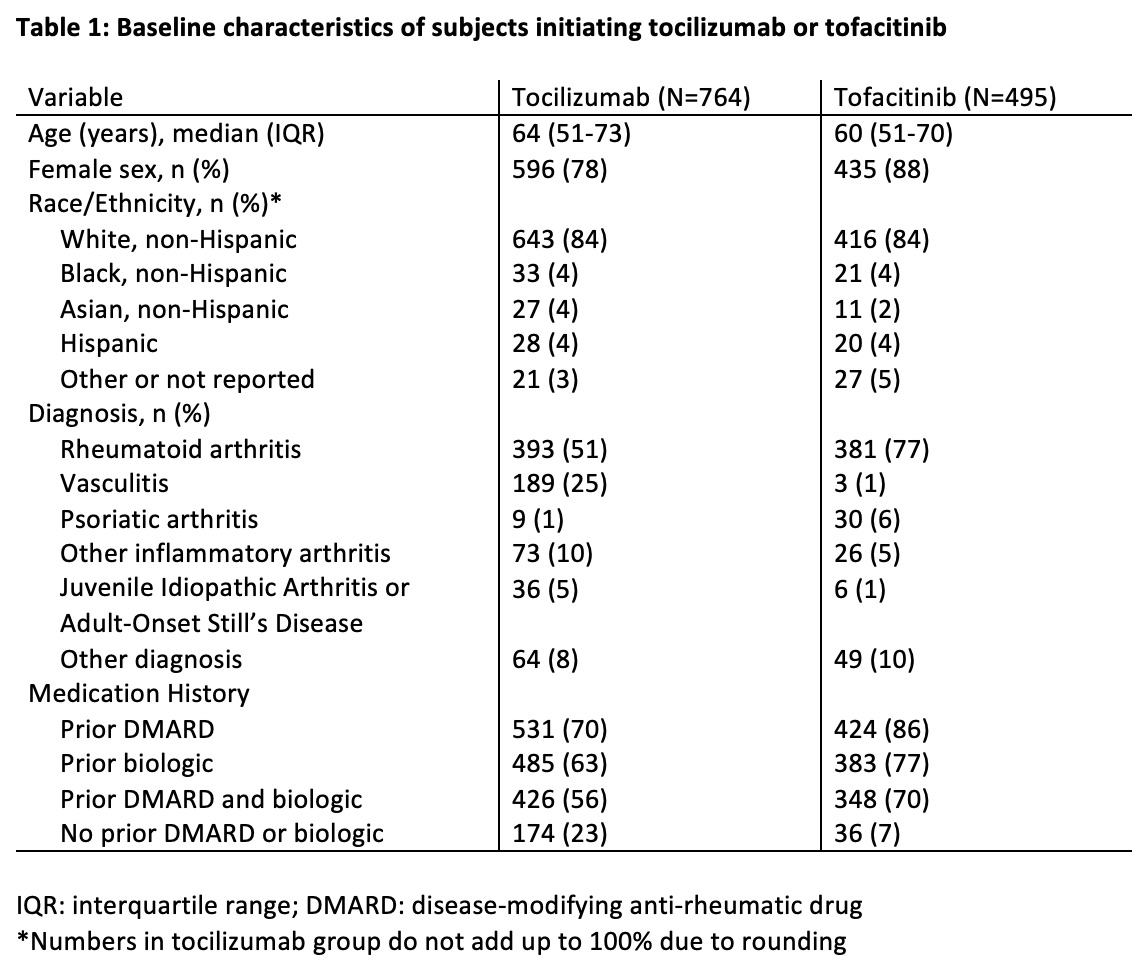 Table 1: Baseline characteristics of subjects initiating tocilizumab or tofacitinib
Table 1: Baseline characteristics of subjects initiating tocilizumab or tofacitinib
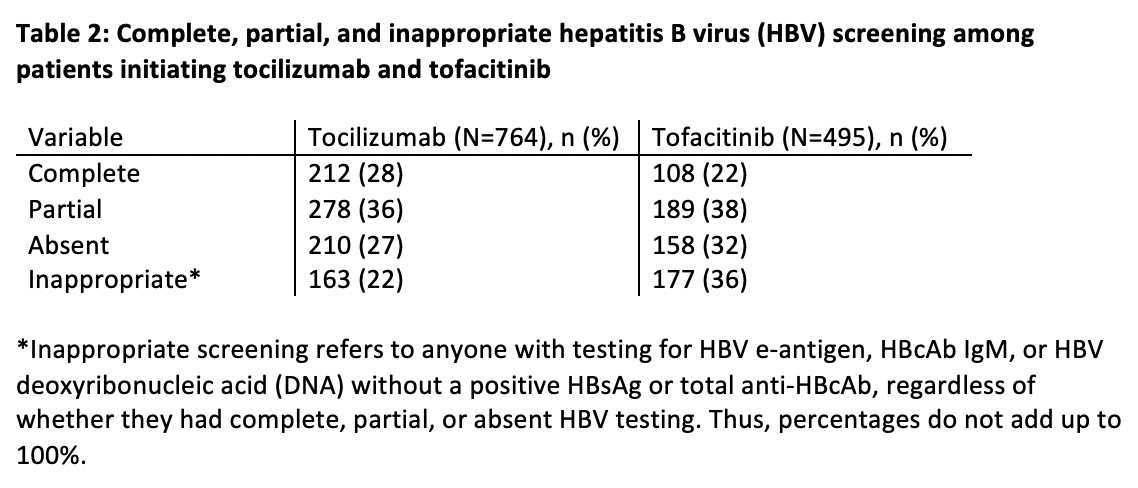 Table 2: Complete, partial, and inappropriate hepatitis B virus (HBV) screening among patients initiating tocilizumab and tofacitinib
Table 2: Complete, partial, and inappropriate hepatitis B virus (HBV) screening among patients initiating tocilizumab and tofacitinib
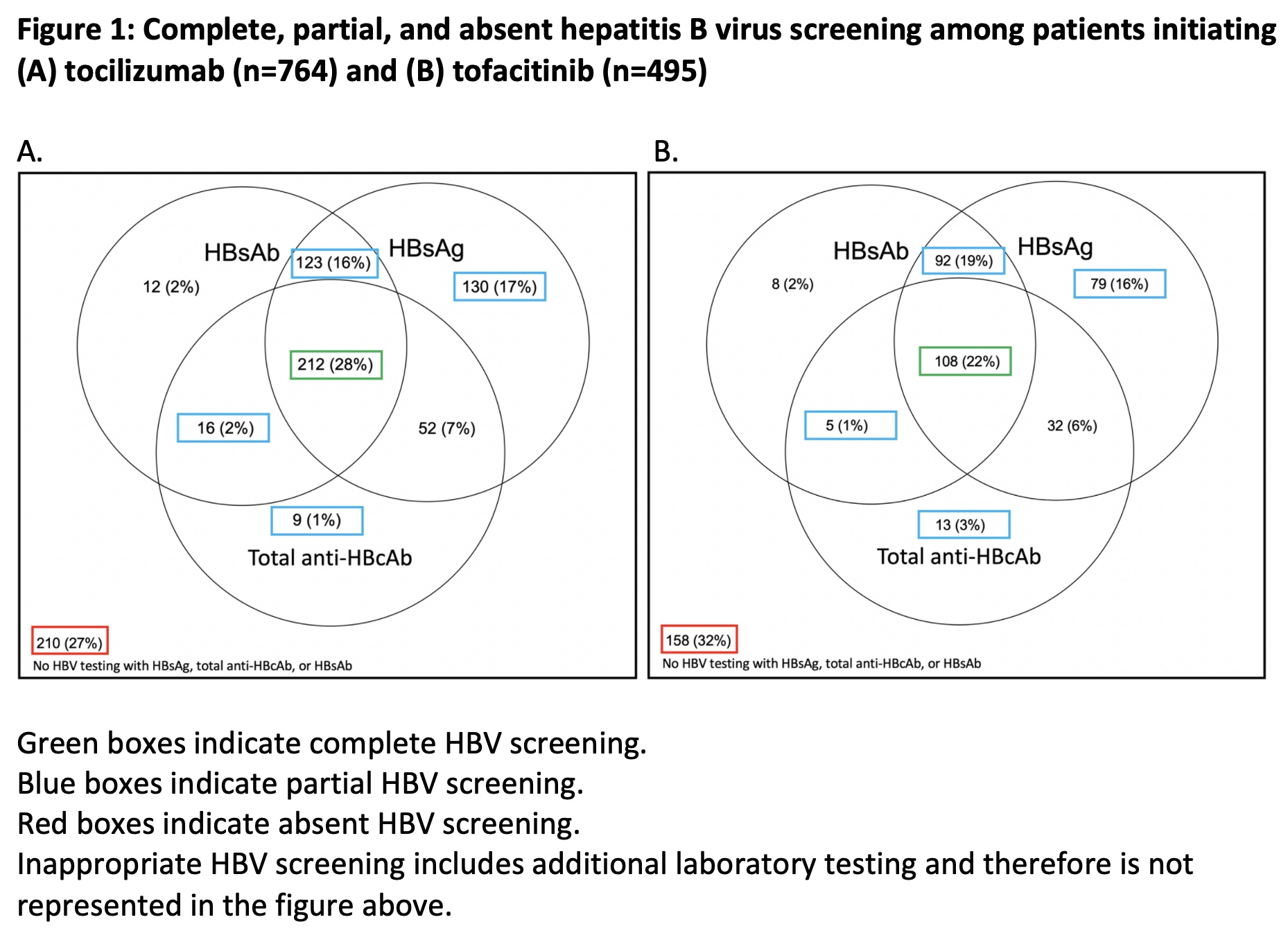 Figure 1: Complete, partial, and absent hepatitis B virus screening among patients initiating (A) tocilizumab (n=764) and (B) tofacitinib (n=495)
Figure 1: Complete, partial, and absent hepatitis B virus screening among patients initiating (A) tocilizumab (n=764) and (B) tofacitinib (n=495)
To cite this abstract in AMA style:
Serling-Boyd N, Mohareb A, Kim A, Hyle E, Wallace Z. Hepatitis B Screening Practices When Prescribing Tocilizumab or Tofacitinib in Real World Practice [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/hepatitis-b-screening-practices-when-prescribing-tocilizumab-or-tofacitinib-in-real-world-practice/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/hepatitis-b-screening-practices-when-prescribing-tocilizumab-or-tofacitinib-in-real-world-practice/
