Session Information
Date: Monday, November 8, 2021
Title: SLE – Diagnosis, Manifestations, & Outcomes Poster III: Outcomes (1257–1303)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Approximately 40% of patients with systemic lupus erythematosus (SLE) develop lupus nephritis (LN), of whom up to 20% may progress to end-stage kidney disease (ESKD).1 Despite this substantial clinical burden, data on healthcare resource utilization (HCRU) and healthcare costs of ESKD among patients with SLE are limited. This study describes HCRU and costs for patients with SLE in the 12 months after ESKD diagnosis in the United States (US).
Methods: This was a retrospective analysis (GSK Study 215295) of two US administrative claims databases (IBM MarketScan [DB#1]; Optum Research Database [DB#2]), conducted between March 2011 and December 2019. The study population comprised adults with SLE who were newly diagnosed with ESKD between March 1, 2012, and December 31, 2018 (diagnosis based on International Classification of Disease [ICD-9/10] diagnosis codes for SLE and ESKD). HCRU and costs (2019 US Dollars) were reported for patients with 12-month continuous enrolment post ESKD diagnosis.
Results: Overall, 1356 and 425 patients with SLE and ESKD were identified in DB#1 and DB#2, respectively. Mean (standard deviation, SD) age was 46.7 (12.32) years (DB#1) and 46.3 (13.95) years (DB#2), and the majority of patients were female (DB#1: 81.8%, DB#2: 79.3%). A total of 805 (DB#1) and 261 (DB#2) patients had 12-month continuous enrollment post-ESKD diagnosis. The mean (SD) annual cost post-ESKD diagnosis was $179,914 ($272,011) (DB#1) and $160,284 ($200,000) (DB#2). Mean (SD) costs were greatest at 1 month post-ESKD diagnosis and stabilized thereafter (Figure). In the 12 months following ESKD diagnosis, HCRU was similar across databases: 97.9% (DB#1) and 96.2% (DB#2) of patients had at least one physician office visit, 68.7% (DB#1) and 71.3% (DB#2) had an inpatient visit, and 47.6% (DB#1) and 55.2% (DB#2) had an emergency room visit (Table 1). SLE-related medications in the 12 months post-ESKD diagnosis included oral corticosteroids (DB#1: 64.8%, DB#2: 74.3%), immunosuppressants (DB#1: 51.7%, DB#2: 58.6%), antimalarials (DB#1: 39.5%, DB#2: 42.2%), and biologics (DB#1: 6.8%, DB#2: 4.6%) (Table 2).
Conclusion: Patients with SLE and newly diagnosed with ESKD had high healthcare costs and utilization 12 months post-ESKD diagnosis. Study results reflect the considerable clinical and economic burden associated with newly diagnosed ESKD among patients with SLE.
Funding: GSK
Reference: 1. Menez SP, et al. Rev Recent Clin Trials 2018;13:105–13
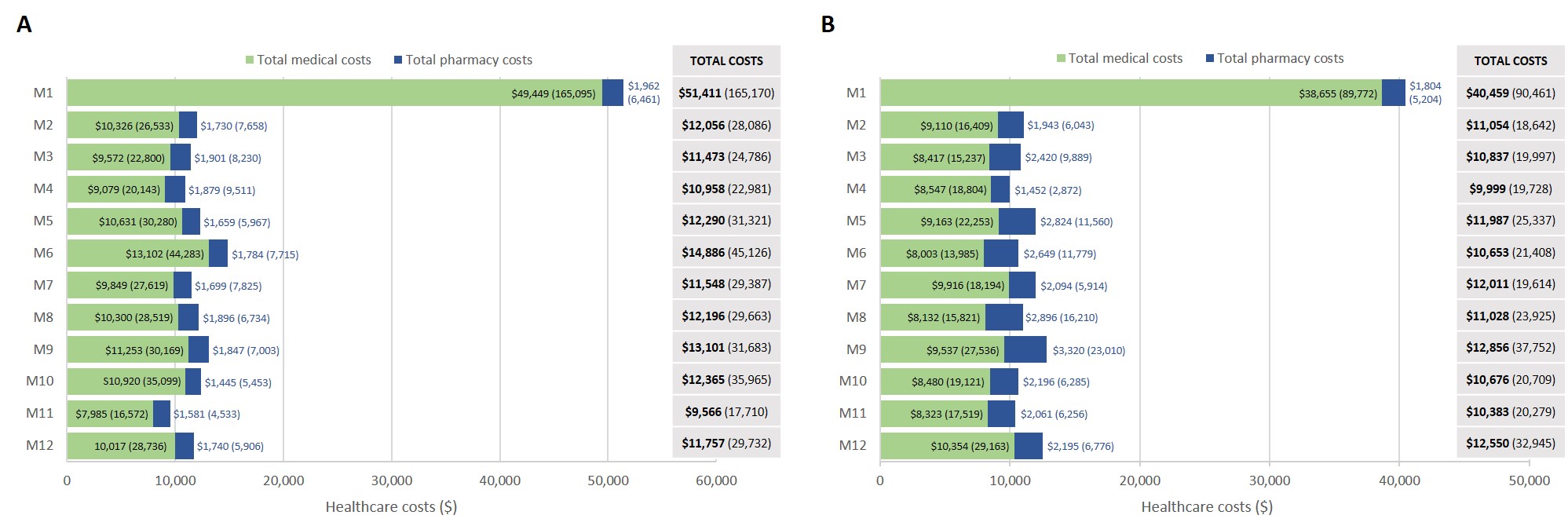 Figure. Mean (SD) healthcare costs (in 2019 US dollars) within 12 months following ESKD diagnosis. A. DB#1 (Nf805), B. DB#2 (Nf261)
Figure. Mean (SD) healthcare costs (in 2019 US dollars) within 12 months following ESKD diagnosis. A. DB#1 (Nf805), B. DB#2 (Nf261)
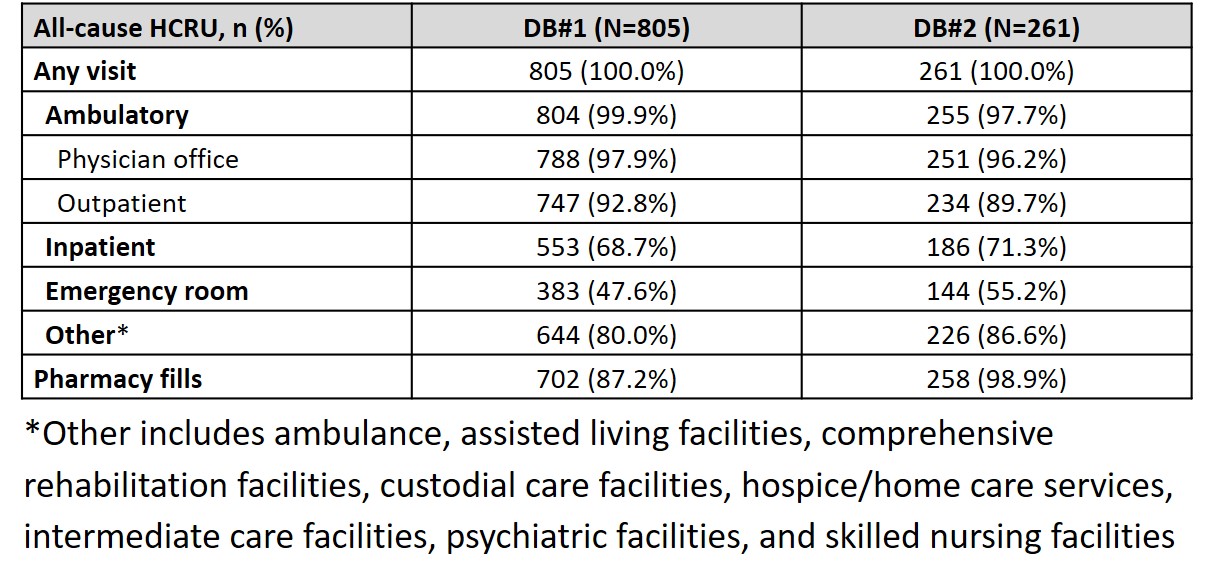 Table 1. HCRU within 12 months following ESKD diagnosis
Table 1. HCRU within 12 months following ESKD diagnosis
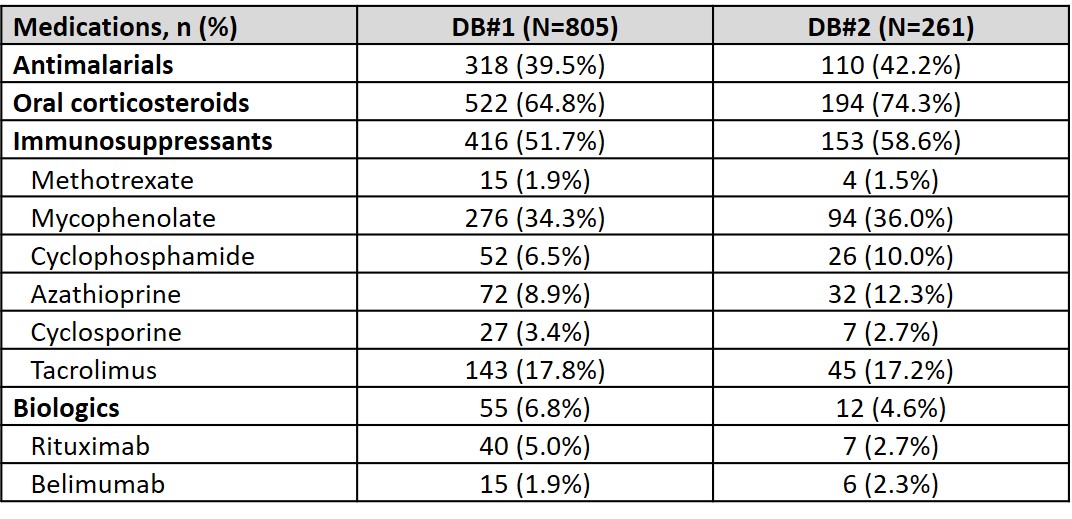 Table 2. SLE-related medications within 12 months following ESKD diagnosis
Table 2. SLE-related medications within 12 months following ESKD diagnosis
To cite this abstract in AMA style:
Huang S, Guisinger A, Bell C. Healthcare Resource Utilization and Costs Associated with Systemic Lupus Erythematosus After Diagnosis of End-Stage Kidney Disease: Evidence from Two United States Databases [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/healthcare-resource-utilization-and-costs-associated-with-systemic-lupus-erythematosus-after-diagnosis-of-end-stage-kidney-disease-evidence-from-two-united-states-databases/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/healthcare-resource-utilization-and-costs-associated-with-systemic-lupus-erythematosus-after-diagnosis-of-end-stage-kidney-disease-evidence-from-two-united-states-databases/
