Session Information
Date: Monday, November 8, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster II: Psoriatic Arthritis I (1329–1363)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Guselkumab (GUS), a selective inhibitor of IL-23, significantly improved the diverse manifestations of active psoriatic arthritis (PsA), including dactylitis and enthesitis, in the DISCOVER (D)-1 & 2 trials of patients (pts) with active PsA1,2, with maintenance of response rates through 1 year.3,4 Dactylitis and enthesitis, extra-articular manifestations of PsA, can be difficult to treat and cause significant disease burden.5,6 Here, we evaluated the ability of GUS to provide long-term resolution of dactylitis and enthesitis in pts with PsA through 2 years of D-2.
Methods: D-2 biologic-naïve pts with active PsA were randomized 1:1:1 to GUS 100 mg every 4 weeks (Q4W); GUS 100 mg at W0, W4, Q8W; or placebo (PBO). At W24, PBO pts crossed over to GUS Q4W. Independent assessors evaluated dactylitis (total score: 0-60) and enthesitis (Leeds Enthesitis Index [LEI]; total score 0-6). These post-hoc analyses assessed baseline (BL) frequency and severity of enthesitis in pts with dactylitis and dactylitis frequency in pts with enthesitis. Post-baseline, changes in dactylitis and LEI scores over time (least squares [LS] mean changes; analysis of covariance [ANCOVA]) and rates of resolution of dactylitis and enthesitis (Chi square correlation test) were determined among pts with these manifestations at BL (missing data imputed as no change/response).
Results: At BL, more D-2 pts had enthesitis (68%) than dactylitis (45%). At BL 78% of pts with dactylitis vs 61% without dactylitis had enthesitis and 51% of pts with enthesitis vs 32% without enthesitis had dactylitis. Among pts with enthesitis at BL, a higher percentage of total pts with dactylitis had severe enthesitis with LEI score ≥3 (52%) versus pts without dactylitis (44%) (Table 1). Among those with the condition at BL, rates of resolution of dactylitis (57% [Q4W], 64% [Q8W]) and enthesitis (44% [Q4W], 54% [Q8W]) at W24 increased through W52 (dactylitis, 74% [Q4W], 78% [Q8W]; enthesitis, 57% [Q4W], 61% [Q8W]) and were maintained at W100 (dactylitis, 72% [Q4W], 83% [Q8W]; enthesitis, 62% [Q4W], 70% [Q8W]). Consistent results were observed when evaluating mean changes in dactylitis and LEI scores and also in pts who crossed over from PBO to GUS Q4W at W24 (Table 2). In pts with both dactylitis and enthesitis at BL, GUS-treated pts showed significant correlations between resolution of enthesitis and dactylitis at W24 (p=0.004), W52 (p< 0.001) and W100 (p=0.039), with nearly 90% of pts with enthesitis resolution also achieving dactylitis resolution at W52 and W100 (Figure).
Conclusion: Pts with PsA often present with concurrent enthesitis and dactylitis, both of which can be recalcitrant to treatment. GUS resolved enthesitis and dactylitis in substantial proportions of pts through W100. GUS-treated pts who achieved enthesitis resolution were more likely to achieve dactylitis resolution and vice versa.
References:
1. Deodhar A et al. Lancet. 2020;395(10230):1115
2. Mease PJ et al. Lancet. 2020;395(10230):1126
3. Ritchlin C et al. RMD Open. 2021;7(1):e001457
4. McInnes IB et al. Arthritis Rheumatol. 2021; 73(4):604
5. Kaeley GS et al. Semin Arthritis Rheum. 2018 48(1):35
6. Mcgonagle D et al. Nat Rev Rheumatol. 2019 15(2):113
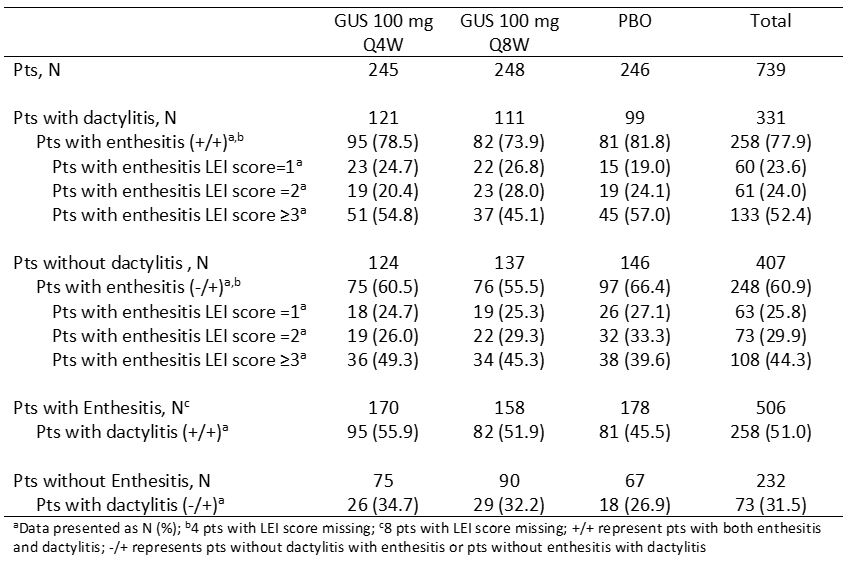 Table 1. Frequency and severity of dactylitis and enthesitis at baseline
Table 1. Frequency and severity of dactylitis and enthesitis at baseline
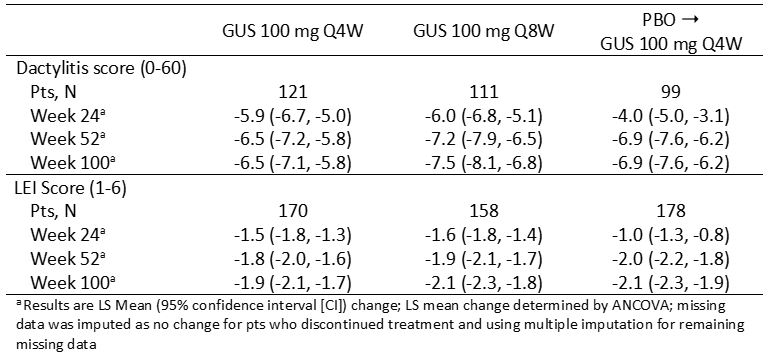 Table 2. LS mean change from baseline over time in dactylitis and LEI scores in pts with manifestation at baseline
Table 2. LS mean change from baseline over time in dactylitis and LEI scores in pts with manifestation at baseline
 Figure. Correlation analysis between resolution of enthesitis and dactylitis over time among pts with enthesitis and dactylitis at baseline
Figure. Correlation analysis between resolution of enthesitis and dactylitis over time among pts with enthesitis and dactylitis at baseline
To cite this abstract in AMA style:
Rahman P, McInnes I, Deodhar A, Schett G, Mease P, Shawi M, Cua D, Sherlock J, Kollmeier A, Xu X, Jiang Y, Sheng S, Ritchlin C, McGonagle D. Guselkumab (TREMFYA®) Maintains Resolution of Dactylitis and Enthesitis in Patients with Active Psoriatic Arthritis: Results Through 2 Years from a Phase 3 Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/guselkumab-tremfya-maintains-resolution-of-dactylitis-and-enthesitis-in-patients-with-active-psoriatic-arthritis-results-through-2-years-from-a-phase-3-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/guselkumab-tremfya-maintains-resolution-of-dactylitis-and-enthesitis-in-patients-with-active-psoriatic-arthritis-results-through-2-years-from-a-phase-3-study/
