Session Information
Date: Monday, November 8, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster II: Psoriatic Arthritis I (1329–1363)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Anemia related to systemic inflammation can be an important feature of psoriatic arthritis (PsA). Hemoglobin (Hgb) levels have been shown to be inversely related to disease activity in other rheumatic diseases.1,2 This post hoc analysis assessed the effect of guselkumab (GUS), a selective IL-23p19 inhibitor, on anemia in the pooled Phase 3 DISCOVER-1 & -2 trials.
Methods: 1120 patients (pts) with active PsA, biologic naïve (except ~30% of DISCOVER-1 pts who had received 1-2 TNF inhibitors), were randomized and treated with SC GUS 100 mg every 4 weeks (Q4W); GUS 100 mg at W0, W4, Q8W; or placebo (PBO, with crossover to GUS 100 mg Q4W at W24). Treatment was given for 1 yr (DISCOVER-1) or 2 yrs (DISCOVER-2). Mean Hgb levels and number (%) of pts with anemia (Hgb < 13.5 g/dL males [M]; < 12.0 g/dL females [F]) were assessed by treatment group through 1 yr. A logistic regression model estimated odds ratios (ORs) and 95% CI for achieving anemia resolution (i.e., anemia at baseline [BL] but not W24). The binary endpoint was anemia responder status at W24 (Y/N); predictors examined included age, sex, swollen/tender joint count, and C-reactive protein (CRP).
Results: The analyses included 1074 pts (96% of pooled study population; 564 M and 510 F). At BL, ~24% of M (N=136) and F (N=120) pts were anemic. Pts with anemia at BL had, on average, more swollen & tender joints, systemic inflammation (CRP), and fatigue (assessed via Functional Assessment of Chronic Illness Therapy-Fatigue [FACIT-F] score) than pts without anemia (Table). For both GUS treatment groups, mean Hgb levels increased from BL through W52 for M and F, particularly among pts who were anemic at BL (Fig 1). For PBO pts, mean Hgb levels were unchanged from BL through W24; however, following PBO®GUS switch at W24, they increased to levels similar to GUS-randomized pts at W52 (Fig 1). Consistently, the proportions of M and F meeting criteria for anemia decreased over time (Fig 2), with M exhibiting a more rapid response (data not shown). Pts with anemia at BL but not at W24 (anemia resolution) comprised more M and had shorter duration of PsA and lower CRP levels at BL than pts with unresolved anemia at W24 (Table). Logistic regression analyses confirmed that F (OR [95% CI]: 0.37 [0.21, 0.65]) and pts with higher BL CRP levels (0.53 [0.41,0.69]) were significantly less likely to achieve anemia resolution at W24 than M and pts with lower BL CRP, respectively. Importantly, pts with anemia resolution (N=112) appeared to exhibit better outcomes at W24 than pts with unresolved anemia (N=136), with numerically fewer mean (SD) swollen (4.8 [6.76] vs 6.2 [8.01]) and tender (10.7 [11.07] vs 12.5 (11.89]) joints, less systemic inflammation (CRP 0.8 [0.92] vs 2.3 [2.73]), and less fatigue (FACIT-F 37.4 [10.60] vs 33.0 [10.45]).
Conclusion: In both M and F pts with active PsA, GUS treatment through 1 yr increased Hgb levels and lessened the prevalence of anemia. Anemia resolution with GUS use, which was more likely in M and pts with lower CRP levels at BL, was associated with improved clinical status relative to pts with persistent anemia.
References:
1. Furst DE, et al. Clin Exp Rheumatol. 2009;27:560-566.
2. Talukdar M, et al. J Clin Diagn Res. 2017;11:EC01-EC04.
 Table. Pt Characteristics by BL Anemia Status and Anemia Responder Status
Table. Pt Characteristics by BL Anemia Status and Anemia Responder Status
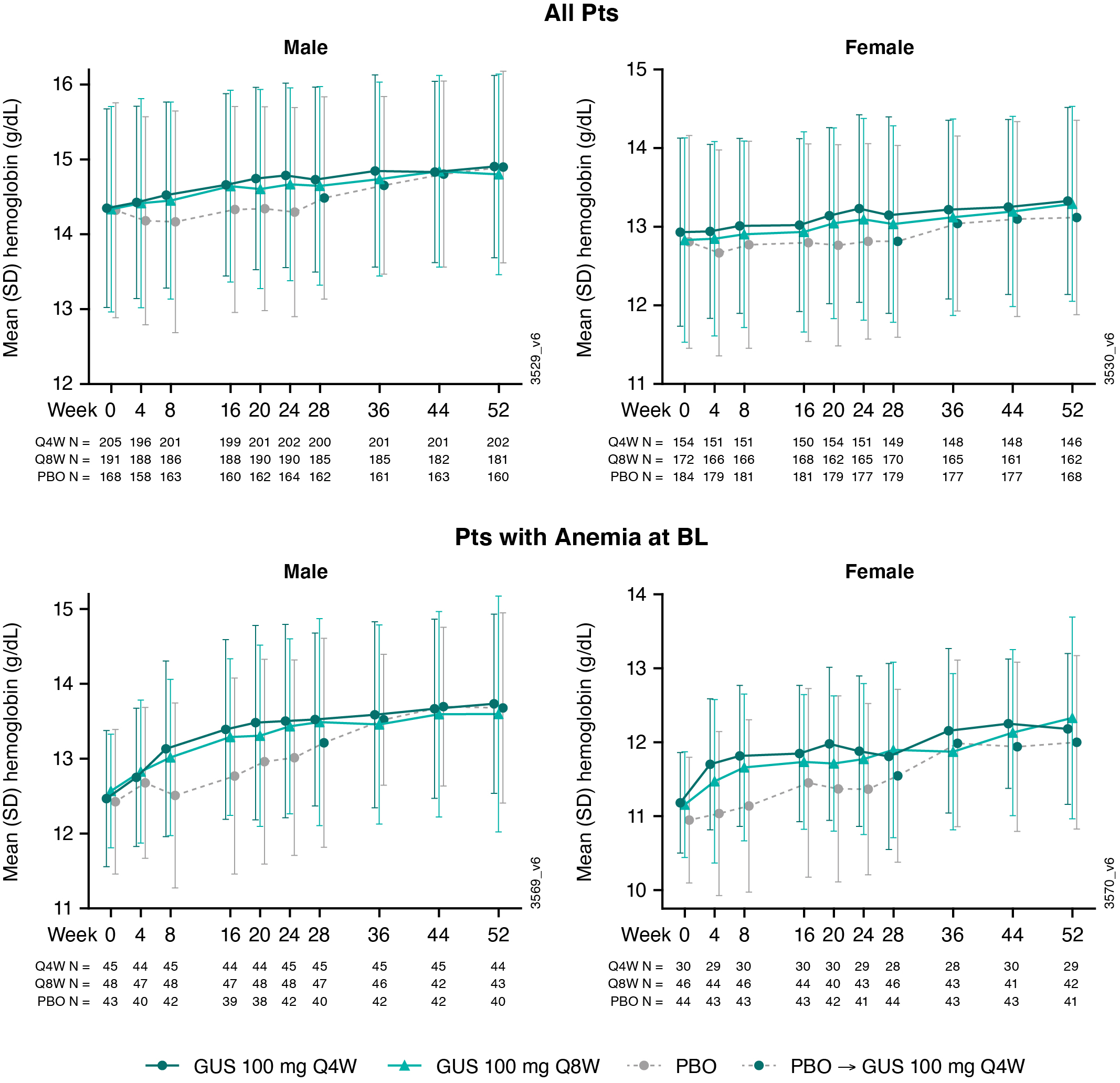 Figure 1. Hgb Levels by Sex Through W52
Figure 1. Hgb Levels by Sex Through W52
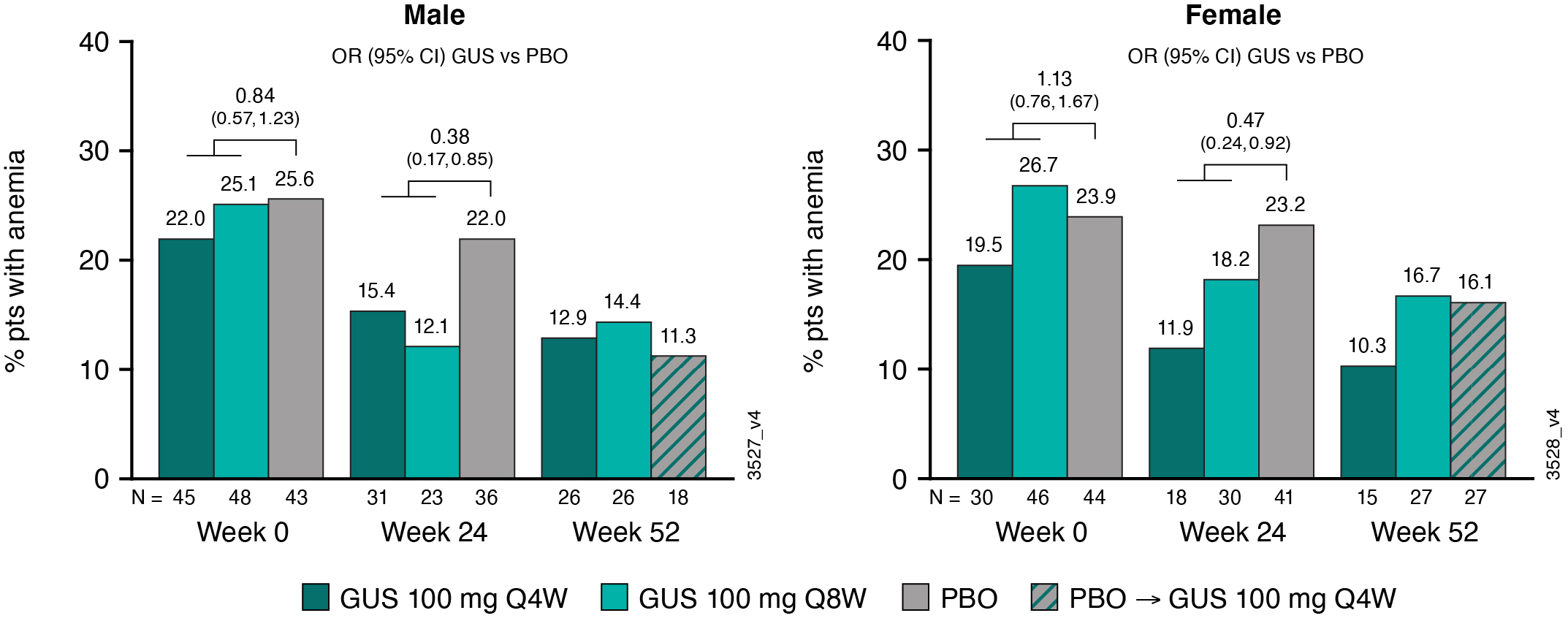 Figure 2. Proportion of Pts With Anemia by Sex and Treatment Group Through W52
Figure 2. Proportion of Pts With Anemia by Sex and Treatment Group Through W52
To cite this abstract in AMA style:
Kavanaugh A, Liu Y, Rahman P, Mease P, Gossec L, Xu X, Hsia E, Shawi M, Han C, Neuhold M, Deodhar A. Guselkumab (TREMFYA®) Improves Anemia in Patients with Active Psoriatic Arthritis: Results from Two Phase 3 Randomized Controlled Clinical Trials [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/guselkumab-tremfya-improves-anemia-in-patients-with-active-psoriatic-arthritis-results-from-two-phase-3-randomized-controlled-clinical-trials/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/guselkumab-tremfya-improves-anemia-in-patients-with-active-psoriatic-arthritis-results-from-two-phase-3-randomized-controlled-clinical-trials/
