Session Information
Date: Tuesday, November 14, 2023
Title: (2227–2256) Spondyloarthritis Including Psoriatic Arthritis – Treatment: SpA Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Guselkumab (GUS) has demonstrated robust efficacy across key PsA domains at Week (W) 24, with effects sustained or further enhanced through 2 years. Timing of clinically meaningful improvement in disease activity (DA), functional status, and health-related quality of life is of interest to PsA patients (pts) and providers. Here we 1) evaluated effect of GUS on time to minimal clinically important improvements (MCII) in pt reported outcomes and composite measures of DA; 2) assessed association between W4/W8 MCII achievement and later disease control (W24/W52).
Methods: This post hoc analysis evaluated 1120 adults with active PsA despite standard therapies from DISCOVER-1 (D1) (swollen & tender joint counts [SJC/TJC] ≥3 each, CRP ≥0.3 mg/dL, 1-2 prior TNF inhibitors [TNFi] in 31% of pts) and D2 (SJC/TJC ≥5 each, CRP ≥0.6 mg/dL, biologic-naïve). Pts were randomized 1:1:1 to GUS 100 mg every 4 weeks (Q4W); GUS 100 mg at W0, W4, then Q8W; or placebo (PBO)®GUS 100 mg Q4W at W24. Time to MCII in outcomes of interest, i.e., improvement in clinical DA Index for PsA (cDAPSA) ≥5.7, Joint Visual Analogue Scale (VAS) ≥15mm, Pt Pain ≥15mm, HAQ-DI ≥0.35, PsA DA Score (PASDAS) ≥0.8, Pt Global Assessment (PtGA) ≥15mm, Skin VAS ≥15mm, Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-F) ≥4, Short Form (SF)-36 physical component summary (PCS) ≥5, was compared between GUS vs PBO with Cox regression adjusting for baseline (BL) levels of respective outcome, treatment group, prior TNFi use, and BL DMARD use. MCII achievement was determined using non-responder imputation and compared between GUS and PBO using logistic regression adjusting for the above covariates. The association between MCII achievement at W4/W8 and stringent clinical response at W24/W52 amongst GUS-treated pts was assessed with logistic regression adjusting for prior TNFi use and BL DMARD use.
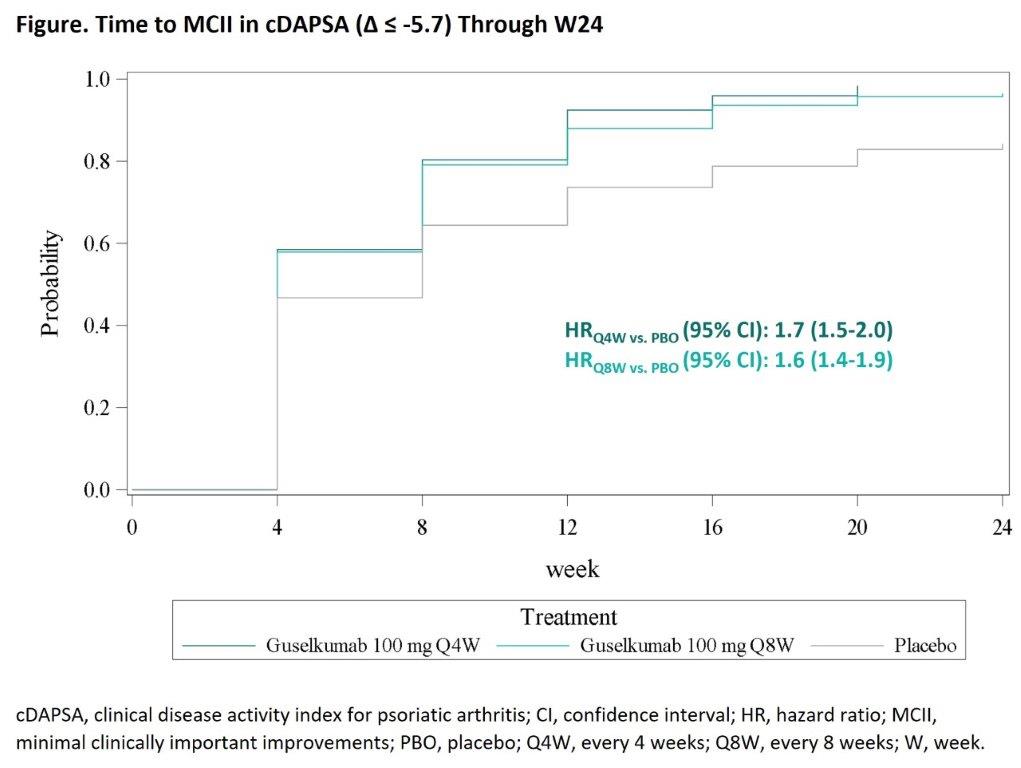
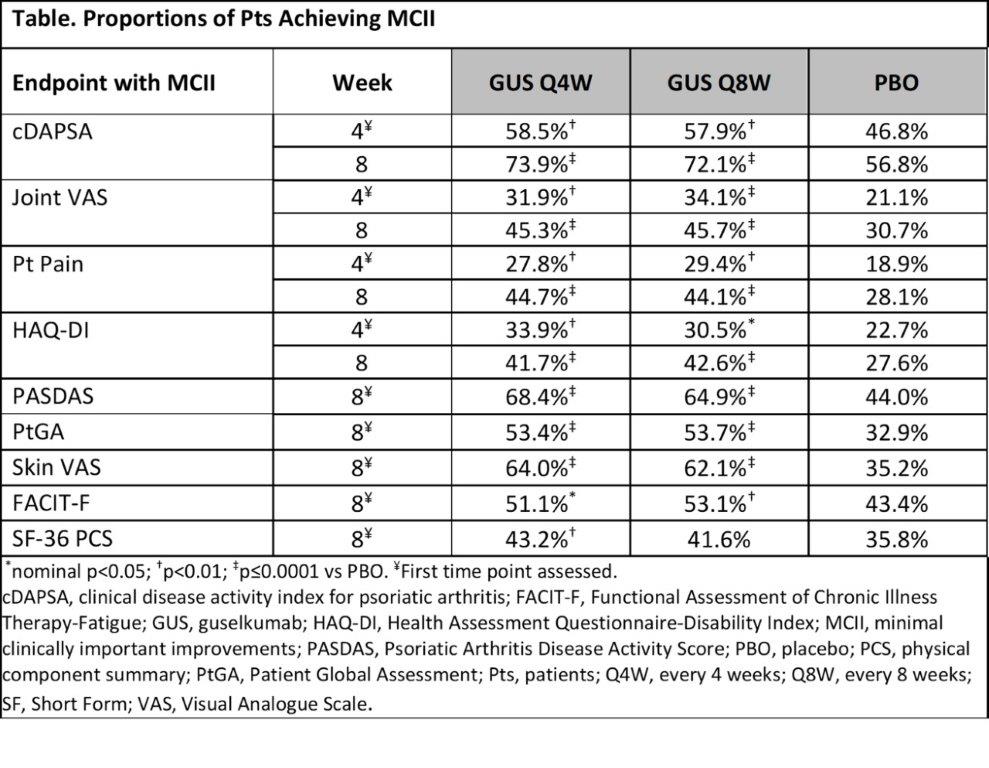
Results: Time to achieve MCII in all studied outcomes was significantly shorter (hazard ratio range: 1.3-2.5; all p< 0.01) for both GUS Q4W and Q8W vs PBO, including cDAPSA as a representative example (Figure), with curve separation occurring at the first timepoint assessed. MCII rates also were significantly higher with GUS vs PBO at the first timepoint assessed, i.e., W4 for cDAPSA, Joint VAS, Pt Pain, and HAQ-DI and W8 for PASDAS, PtGA, Skin VAS, FACIT-F, and SF-36 PCS (Table). W4 achievement of MCII in cDAPSA, Joint VAS, Pt Pain, and HAQ-DI was associated with greater odds of achieving future disease control, defined as ACR50, ACR70, cDAPSA ≤13, PASDAS ≤3.2, and minimal DA (MDA), at W24 (odds ratio [OR]: 1.5-3.6) and W52 (OR: 1.2-2.4). W8 achievement of MCII in all studied outcomes was also associated with greater odds of achieving disease control at W24 (OR: 1.4-17.2) and W52 (OR: 1.2-5.4).
Conclusion: In pts with active PsA, treatment with GUS was associated with more rapid MCII in both clinical and pt reported outcomes compared with PBO. As early as W4, achievement of MCII vs non-achievement in each studied outcome, particularly PASDAS and cDAPSA, was associated with greater odds of longer-term stringent DA control. Findings highlight the impact of these rapid improvements with GUS (as early as W4) on the trajectory of long-term pt outcomes.
To cite this abstract in AMA style:
Curtis J, Deodhar A, Soriano E, Rampakakis E, Shawi M, shiff N, Strauss M, Han C, Tillett W, Gladman D. Guselkumab Provides Rapid Clinically Meaningful Improvements in Clinical and Patient Reported Outcomes and Sustained Disease Control of Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/guselkumab-provides-rapid-clinically-meaningful-improvements-in-clinical-and-patient-reported-outcomes-and-sustained-disease-control-of-psoriatic-arthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/guselkumab-provides-rapid-clinically-meaningful-improvements-in-clinical-and-patient-reported-outcomes-and-sustained-disease-control-of-psoriatic-arthritis/