Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: The Phase 3b COSMOS study (NCT03796858) demonstrated the efficacy and safety of guselkumab (GUS), an IL-23 p19 subunit inhibitor, in patients (pts) with psoriatic arthritis (PsA) who had inadequate response (IR; insufficient efficacy or intolerance) to 1–2 tumor necrosis factor inhibitors (TNFi).1 The purpose of this analysis is to assess the efficacy of GUS 100 mg Q8W in TNFi-IR pts through 1 year by using and comparing 6 multi-domain composite indices validated in PsA.
Methods: In total, 285 pts (189 GUS, 96 placebo [PBO]) were enrolled in COSMOS; mean age was 49 years and mean disease duration was 8.4 years.1 Pts who received PBO crossed over to GUS at either Week (W) 16 (early escape, n=45/96) or W24 (planned, n=51/96). PsA Disease Activity Score (PASDAS), GRAppa Composite scorE (GRACE), Disease Activity Index for PsA (DAPSA), modified Composite Psoriatic Disease Activity Index (mCPDAI, excludes BASDAI), PsA Response Criteria (PsARC), and Minimal Disease Activity (MDA) were analyzed (thresholds as published2,3). As-observed data are presented at baseline (BL), W24 and W48, without imputation of missing data.
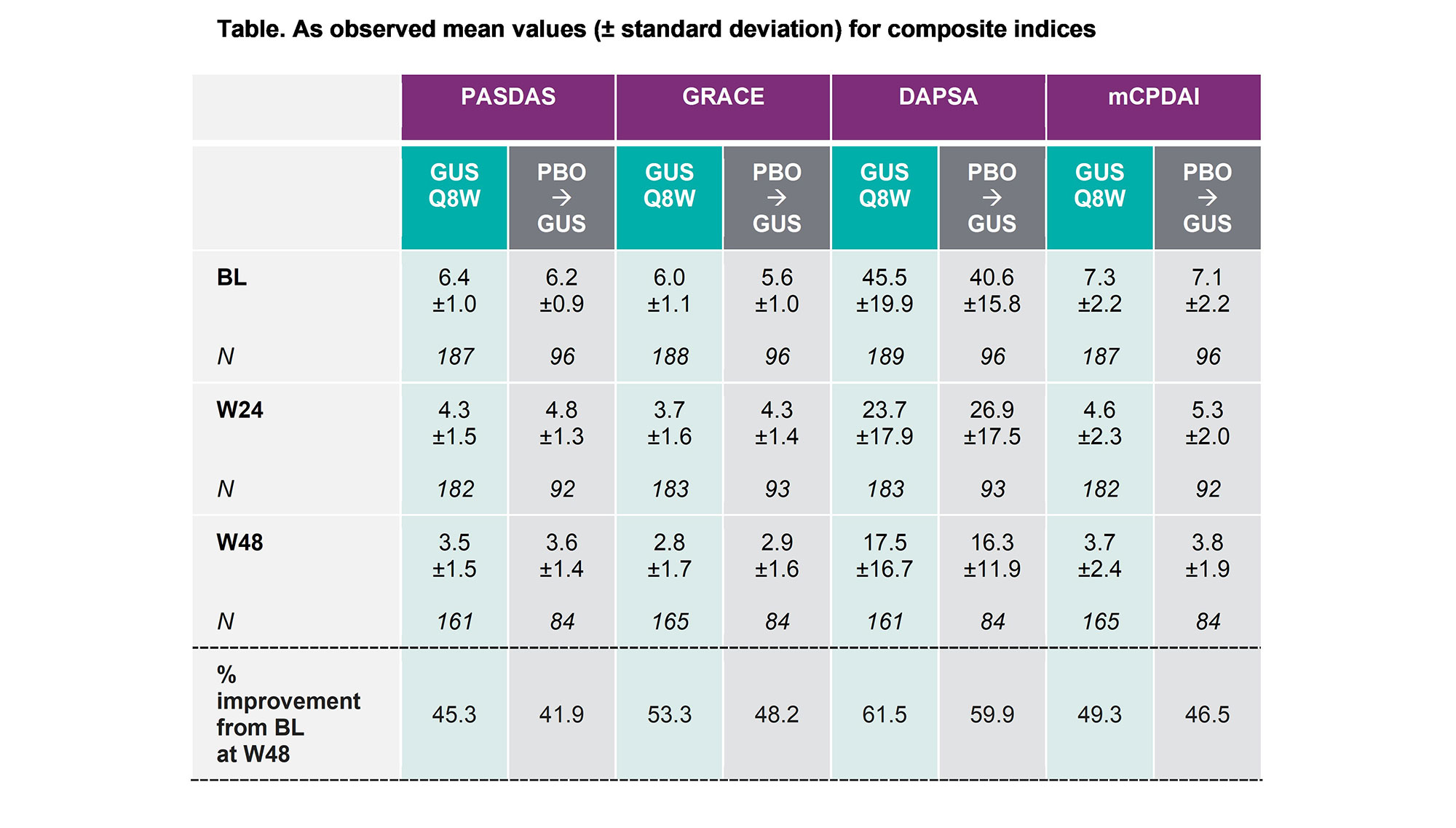
Results: Overall, 167/189 (88%) GUS and 83/96 (86%) PBOàGUS pts completed the study through W44. Pts had active disease, with similar BL mean values for PASDAS, GRACE, DAPSA and mCPDAI between GUS and PBO groups (Table). Across these indices, GUS pts demonstrated robust improvements (45–62%) in mean scores from BL to W48 (Table). PBO pts who crossed over to GUS at W16 or W24 also showed rapid improvement in their index scores, with mean values and % improvement at W48 consistent with those observed in pts randomized to GUS at BL (Table).
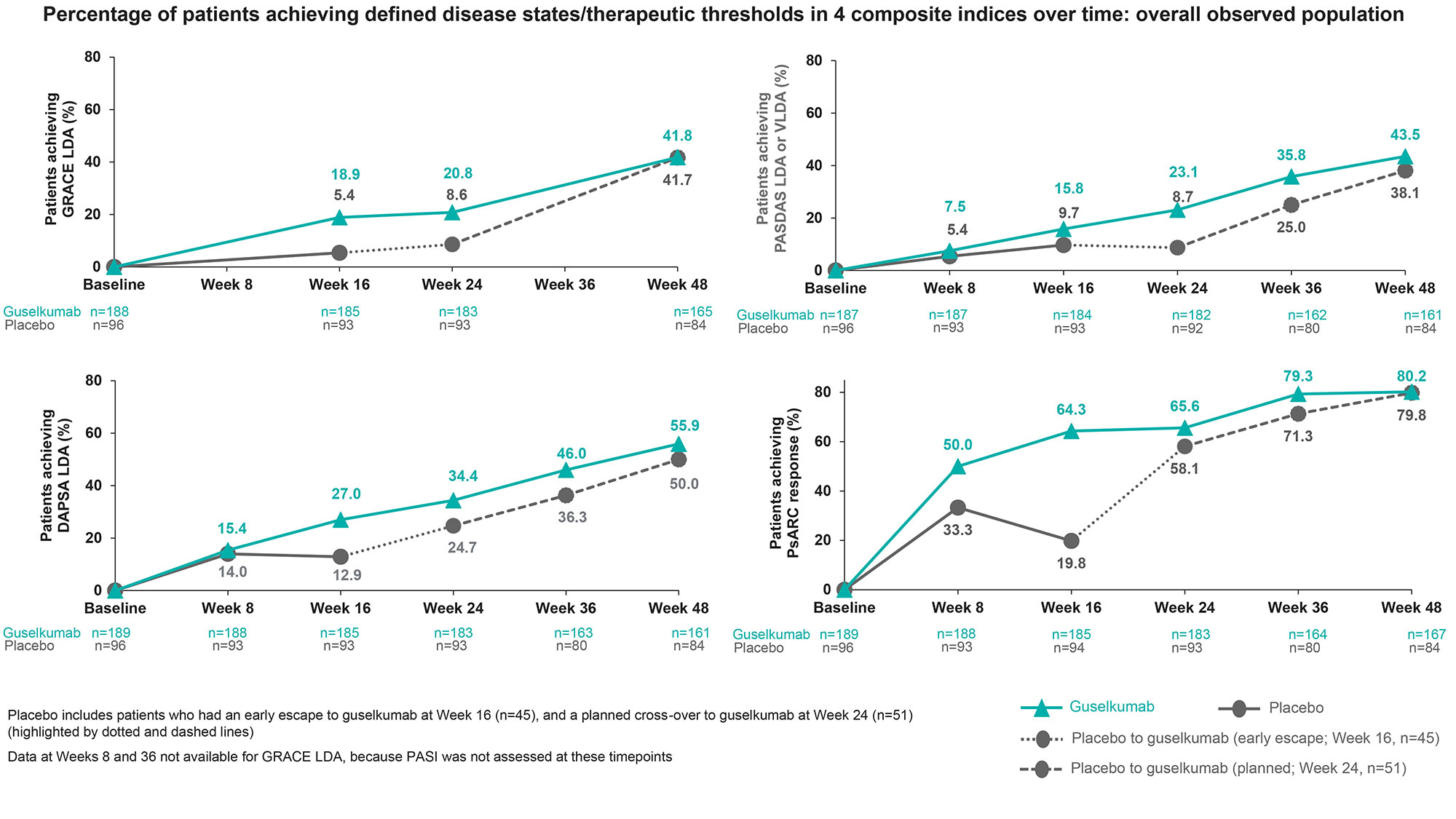
Among the 4 indices with defined disease states/therapeutic thresholds for low disease activity (LDA) (Figure), those focusing on joints (PsARC [4 components] and DAPSA [5 components]) resulted in the highest response rates (range, 50–80%); LDA according to GRACE (8 items, including skin) and PASDAS (8 items, focus on musculoskeletal with no skin assessment) was attained less frequently (range, 38–44%). Using the PsARC, 50% of GUS pts responded as early as W8. Response rates among GUS-treated pts generally did not plateau by W48 (Figure). At W48, 33% and 30% of GUS and PBOàGUS pts, respectively, achieved a status of MDA.
Conclusion: GUS provided robust and substantial benefits to pts with active TNFi-IR PsA across multiple domains. Importantly, rates of achieving low levels of disease activity continued to increase through the duration of the study without an observable plateau at W48. Thresholds for DAPSA and PsARC response were easier to achieve than comprehensive indices with more domains that are required to improve simultaneously (eg GRACE, PASDAS, MDA). GUS performed well regardless of the focus of the composite indices (joints, skin, enthesitis, dactylitis, or patient-reported outcomes). Together, these findings support the role of GUS as an effective treatment option for the diverse domains of PsA.
References
1. Coates LC et al. Ann Rheum Dis 2021. doi: 10.1136/annrheumdis-2021-220991
2. Helliwell PS et al. Arthritis Care Res 2020;72:1579–88
3. Clunie G et al. Rheumatol Adv Pract 2018;2(2): doi: 10.1093/rap/rky042
To cite this abstract in AMA style:
Gossec L, Sharaf M, Theander E, Neuhold M, Bergmans P, Shawi M, Perate M, Contré C, Coates L. Guselkumab Efficacy in Psoriatic Arthritis Assessed by Multi-domain Composite Indices: Data from the Phase 3b COSMOS Trial in a TNFi-IR Population [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/guselkumab-efficacy-in-psoriatic-arthritis-assessed-by-multi-domain-composite-indices-data-from-the-phase-3b-cosmos-trial-in-a-tnfi-ir-population/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/guselkumab-efficacy-in-psoriatic-arthritis-assessed-by-multi-domain-composite-indices-data-from-the-phase-3b-cosmos-trial-in-a-tnfi-ir-population/