Session Information
Date: Monday, November 18, 2024
Title: Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Anti-inflammatory prophylaxis is recommended during the initial period of urate-lowering therapy (ULT) as gout flares are common during this time. The aim of this study was to determine the frequency of gout flares following discontinuation of prophylaxis during the early phase of ULT.
Methods: A literature review and meta-analysis were undertaken. Eligibility criteria included: any clinical trial of people with gout starting ULT with co-administration of anti-inflammatory prophylaxis, and the percentage of participants with ≥ one flare was reported both during and after prophylaxis. Data including number of participants, sex, age, type/dose of ULT, and type/duration of prophylaxis were collected. The percentage of participants having ≥ one flare was collected just prior to stopping prophylaxis; within three months of stopping prophylaxis; and the last period of the trial. Where data were not reported, an invitation was sent to the study investigators requesting aggregated unpublished data. Random effects meta-analyses were used to generate pooled estimates of the percentage of participants experiencing ≥ one flare and changes in these percentages for the studies reporting the relevant paired time periods. Sensitivity analyses based on anti-inflammatory prophylaxis duration, trial duration, ULT class, and excluding placebo arms were undertaken.
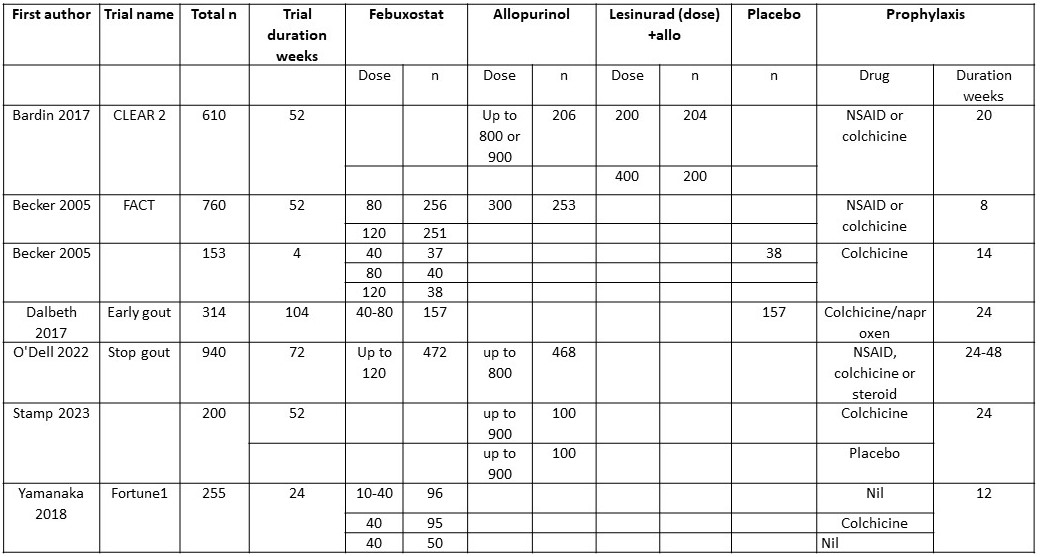
Results: A total of 422 trials were identified. Six were included in the final analysis, together with aggregated, unpublished data from the Veterans Affairs STOP Gout trial giving a total of seven studies including 2972 participants. Trial duration ranged from 24 to 104 weeks and prophylaxis from 8 to 48 weeks( Table 1).
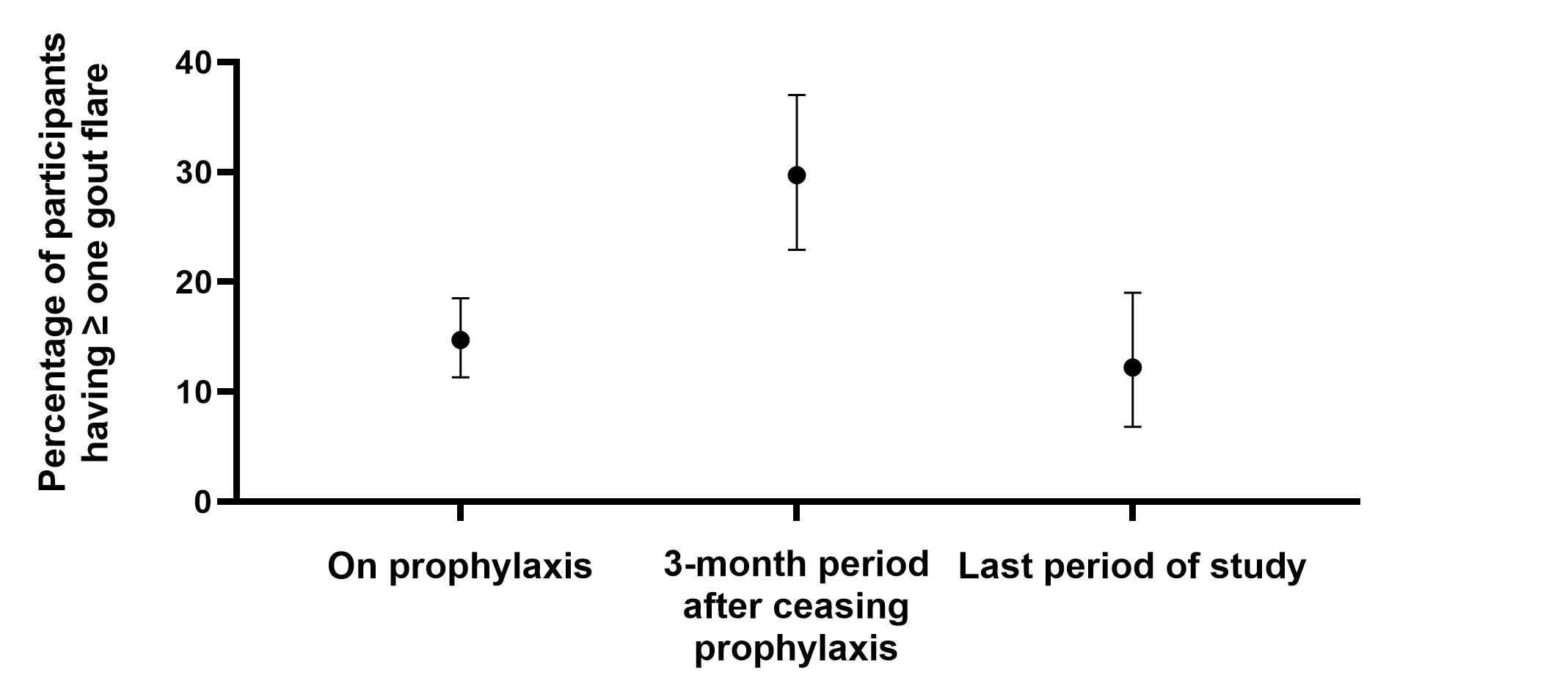
During the period on prophylaxis, the pooled random effects estimate of the percentage of participants having ≥ one flare was 14.7% (95%CI 11.3-18.5; I2=83.6%). During the three-month period after ceasing prophylaxis, the pooled random effects estimate of the percentage of participants having ≥ one flare was 29.7% (95%CI 22.9-37.0; I2=92.4%). During last period of the study, the pooled random effects estimate of the percentage of participants having ≥ one flare was 12.2% (95%CI 6.8-19.0; I2=93.6%) (Figure). There was a statistically significant mean difference in the percentage of participants having ≥ one flare while on prophylaxis and soon after ceasing prophylaxis (mean difference -16.0% (95%CI -18.2 to -13.8); p< 0.0001; I2 86.6%) (Figure 1). There was also a statistically significant mean difference in the percentage of participants having ≥ one flare soon after ceasing prophylaxis compared to the last period of the study (mean difference 16.0% (95%CI 9.2 to 22.9); p< 0.001; I2 88.1%). Despite considerable heterogeneity in the results, sensitivity analyses indicated no material effects of prophylaxis duration, trial duration, ULT class, or placebo on the results.
Conclusion: Gout flares are common after stopping prophylaxis, irrespective of duration, but return to levels seen during prophylaxis. People with gout should be cautioned about risk of flares and have a flare management plan particularly in the three months following discontinuation of anti-inflammatory prophylaxis.
To cite this abstract in AMA style:
Stamp L, Frampton C, O'Dell J, Mikuls T, Newcomb J, Dalbeth N. Gout Flares After Stopping Anti-inflammatory Prophylaxis During the Early Phases of Urate-Lowering Therapy [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/gout-flares-after-stopping-anti-inflammatory-prophylaxis-during-the-early-phases-of-urate-lowering-therapy/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/gout-flares-after-stopping-anti-inflammatory-prophylaxis-during-the-early-phases-of-urate-lowering-therapy/