Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Avacopan, a C5a receptor inhibitor, has emerged as a novel treatment for ANCA-associated vasculitis (AAV), providing an alternative to glucocorticoids1. Despite its promising potential, severe liver dysfunction associated with avacopan has been reported in Japan2,3. Given the relatively recent approval of avacopan, there is a lack of real-world evidence detailing its adverse events. We thus sought to elucidate the clinical differences between patients with and without avacopan.
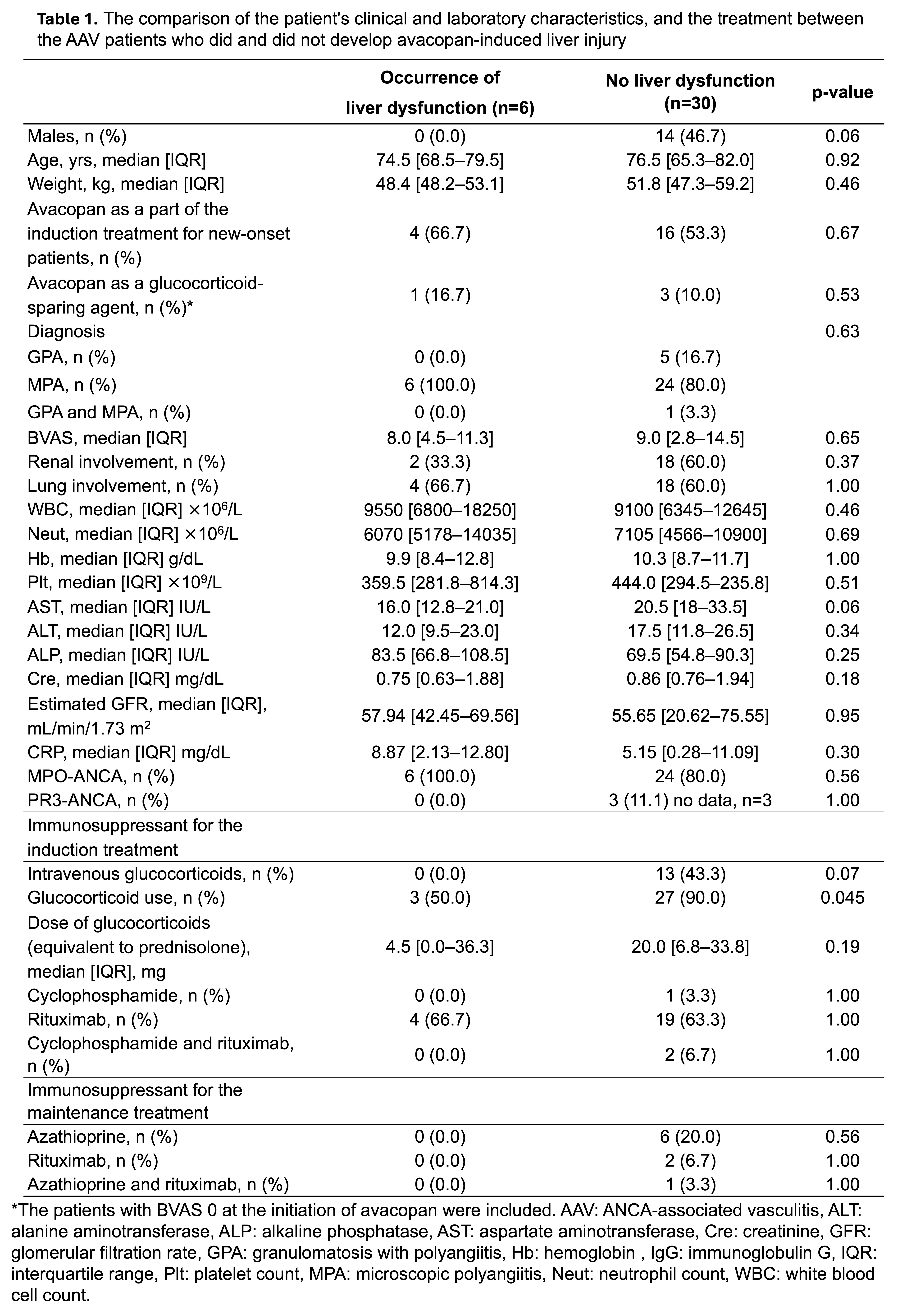
Methods: The AAV patients treated with avacopan from June 2022 to December 2023 were enrolled. We classified the patients using the 2022 ACR/EULAR classification criteria for granulomatosis with polyangiitis, microscopic polyangiitis, and eosinophilic granulomatosis with polyangiitis and compared the baseline characteristics, BVAS version 3, and immunosuppressive treatment between the patients who developed hepatotoxicity due to avacopan and those who did not. Avacopan-induced hepatotoxicity was defined as serum aspartate aminotransferase or alanine aminotransferase >5× the upper limit of normal (ULN) or alkaline phosphatase >2× the ULN due to avacopan as referenced in the American Association for the Study of Liver Diseases practice guidance on drug, herbal, and dietary supplement-induced liver injury.
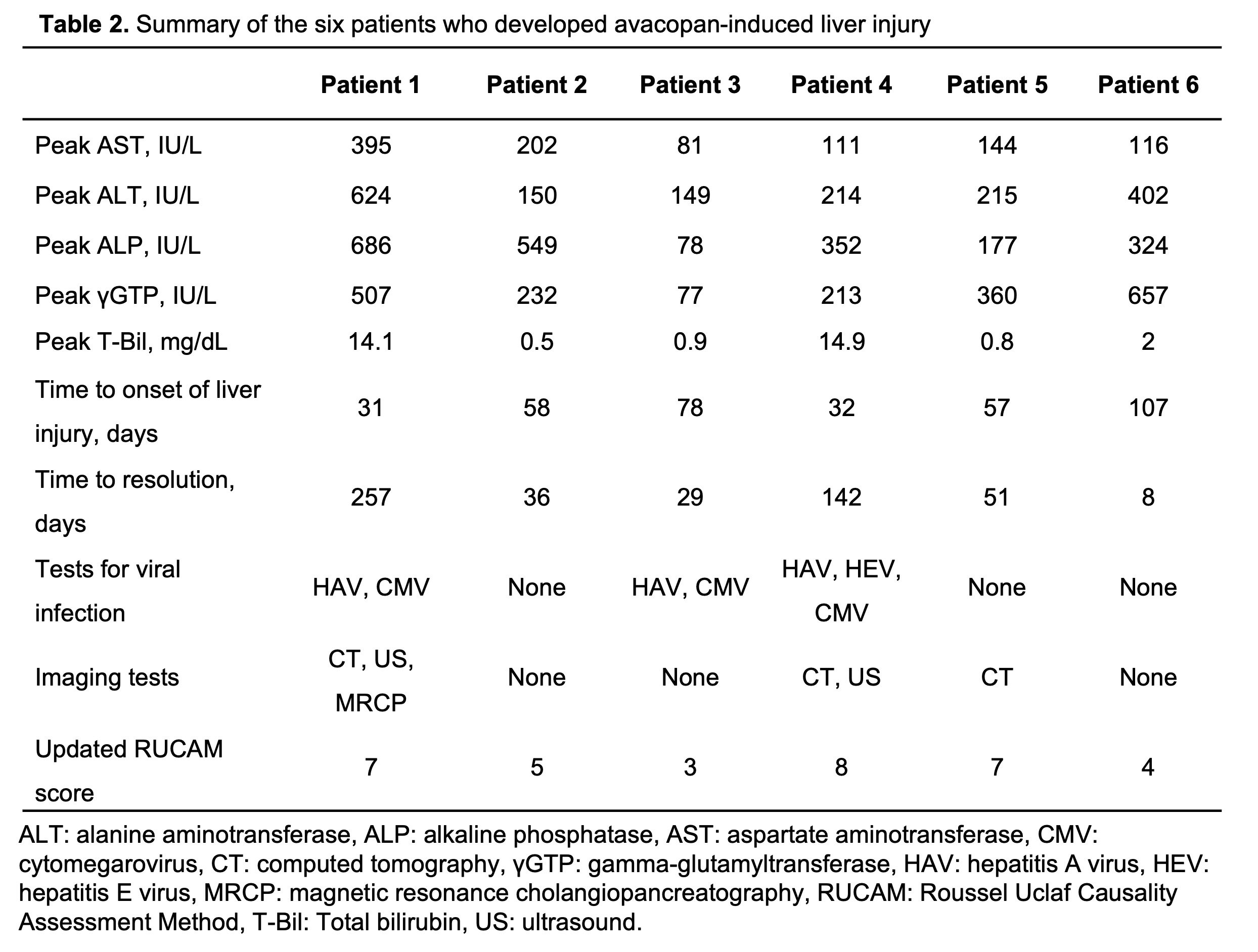
Results: Thirty-six patients were included in the study. Six patients (16.7%) developed liver abnormalities due to avacopan; their liver dysfunction findings are summarized in Table 1. These six patients had no liver abnormalities, history of viral hepatitis, other liver diseases, or alcohol use disorder before the initiation of avacopan. They were negative for hepatitis C, and none had a chronic hepatitis B virus (HBV) infection. HBV reactivation was not observed in the patients with prior HBV exposure. Table 2 provides the results of other viral laboratory tests and imaging tests performed in relation to liver damage, which were negative and varied among the patients. Two of the six patients had severe drug-induced liver injury (DILI) with jaundice, and one of the two was diagnosed with vanishing bile duct syndrome on liver biopsy. After cessation of avacopan, all six patients showed improvement in liver function. Significant differences in glucocorticoid use were observed between the patients who developed avacopan-induced hepatotoxicity and those who did not (p=0.045).
Conclusion: Our findings suggest a potential association between the absence of glucocorticoid use concomitant with avacopan and DILI associated with avacopan.
References:
1. Jayne DRW, et al. N Engl J Med 2021;384:599–609.
2. Yamaguchi S, et al. Rheumatology 2023:kead285.
3. Kojima K, et al. Rheumatology 2023:kead509.
To cite this abstract in AMA style:
Uchida T, Fukui S, Iwamoto N, Umetsu A, Okamoto M, Fujikawa K, Mizokami A, Tomokawa T, Hara K, Horai Y, Kawakami A. Glucocorticoids May Mitigate the Risk of Avacopan-Induced Liver Dysfunction in ANCA-Associated Vasculitis: Data from a Multicenter Observational Study in Japan [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/glucocorticoids-may-mitigate-the-risk-of-avacopan-induced-liver-dysfunction-in-anca-associated-vasculitis-data-from-a-multicenter-observational-study-in-japan/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/glucocorticoids-may-mitigate-the-risk-of-avacopan-induced-liver-dysfunction-in-anca-associated-vasculitis-data-from-a-multicenter-observational-study-in-japan/