Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Glucocorticoids are frequently used as bridging therapy for rheumatoid arthritis (RA) patients starting conventional synthetic DMARDs. Both EULAR and ACR guidelines (1,2), recommend to use the lowest effective dose for the shorter duration possible, ideally less than three months. In addition, glucocorticoids are not recommended when starting a b/tsDMARD. We aim to assess glucocorticoid usage after treatment initiation with bDMARDs and JAKi in RA patients in real life.
Methods: Data on patients with RA switching from a conventional synthetic DMARD to a first biologic or JAKi enrolled in BIOBADASER 3.0 (a multicenter Spanish Registry on Adverse Events of Advanced Therapies in Rheumatic Diseases) were analyzed. Biologic treatment included TNFi, anti-IL6, anti-CD20, and CTLA4-Ig. Patients with any biologic or JAKi, all in combination with glucocorticoids at therapy initiation were included. As TNFi was the larger group, a random sample was taken for comparison purposes. Descriptive statistics were used to calculate the frequency and average daily glucocorticoid usage according to therapy group, both at baseline and 1 year after treatment initiation. Wilcoxon Singed Rank test was used to compare glucocorticoid dose changes from baseline to 1 year after initiation of the b/tsDMARDs and ANOVA, to compare the mean dose differences across groups.
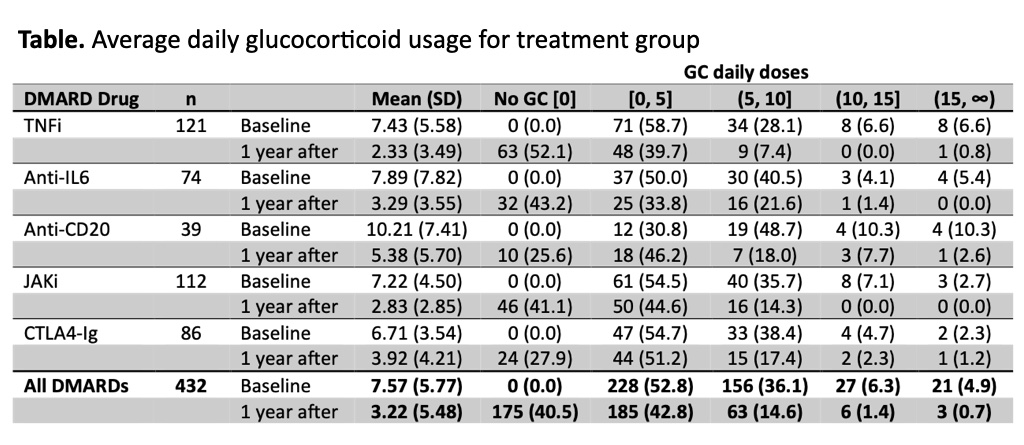
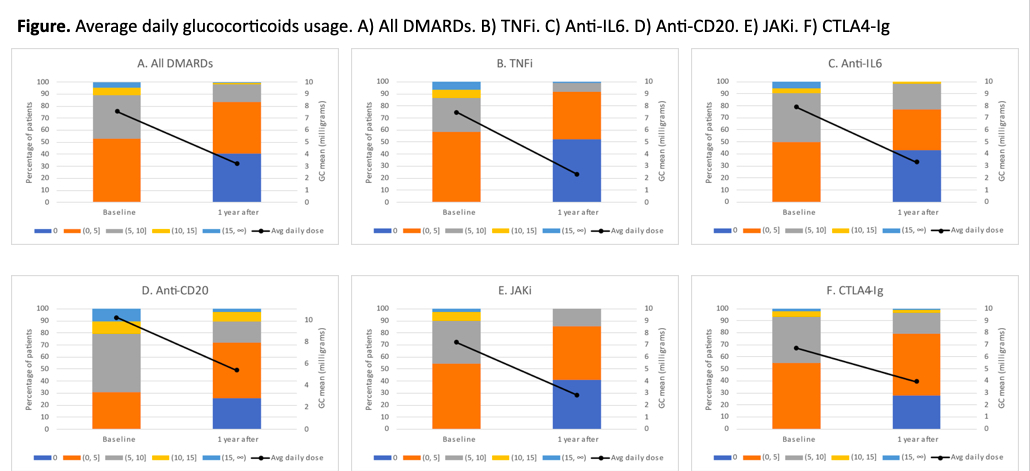
Results: A total of 432 RA patients met the inclusion criteria and were included in the analysis, 78.2% women, mean age 58.4 (12.2) years old, median disease duration 5.2 years [IQR: 2.1-10.8] and mean DAS28-ESR at baseline 4.72 (1.33). Mean (SD) glucocorticoid daily dose decreased from 7.6 mg (5.8) at baseline to 3.2 (5.5) mg at 1-year follow-up (Figure). Daily dose changes were statistically significant (Wilcoxon Signed Rank test: p < 0.001). Mean difference from baseline to one year follow-up was 5.13 for TNFi, 4.59 for anti-IL6, 4.83 for anti-CD20, 4.38 for JAKi, and 2.78 for CTLA4-Ig (ANOVA: p=0.12). The proportion of patients with a decrease in glucocorticoids dose from baseline to one year follow-up after b/tsDMARD initiation was 72.7% for TNFi, 62.2% for anti-IL6, 66.7% for anti-CD20, 67.9% for JAKi, and 60.5% for CTLA4-Ig, and the proportion of patients with no glucocorticoids at one year was 52.1%, 43.2%, 25.6%, 41.1% and 27.9% respectively for each treatment (Table).
Conclusion: Our data shows that glucocorticoids are frequently used and for longer than 3 months when initiating b/tsDMARDs. Around 2% of patients have glucocorticoid doses over 10mg and 15% over 5mg after one year follow-up. Further studies are necessary to evaluate variables associated with persistent use of glucocorticoids. References:
- Smolen JS, et al. Ann Rheum Dis 2023;82:3–18.
- Fraenkel, L. Arthritis Care & Research 2021; 73: 924–939
To cite this abstract in AMA style:
Otero-Valera L, Alvaro-Gracias J, Calvo J, Campos C, Garcia Dorta A, Sánchez-Alonso F, Castrejon I. Glucocorticoid Use in Rheumatoid Arthritis Patients Initiating Biological or Targeted Synthetic Disease Modifying Anti-rheumatic Drugs (b/tsDMARDs) in Real Life: Data from BIOBADASER [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/glucocorticoid-use-in-rheumatoid-arthritis-patients-initiating-biological-or-targeted-synthetic-disease-modifying-anti-rheumatic-drugs-b-tsdmards-in-real-life-data-from-biobadaser/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/glucocorticoid-use-in-rheumatoid-arthritis-patients-initiating-biological-or-targeted-synthetic-disease-modifying-anti-rheumatic-drugs-b-tsdmards-in-real-life-data-from-biobadaser/