Session Information
Date: Sunday, November 13, 2022
Title: Vasculitis – ANCA-Associated Poster II: Treatment Efficacy, Clinical Outcomes, Biomarkers
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Although the 2021 ACR/VF guidelines for ANCA-associated vasculitis (AAV) recommend reduced-dose glucocorticoids (GCs) over standard-dose GCs (1), this approach still leads to substantial toxicity, e.g., skeletal, metabolic, cardiovascular, psychological, and infectious adverse effects. Studies have demonstrated that, compared to non-use, low-dose GCs (5-15 mg prednisone daily) increase the risk of cardiovascular disease 3.4-fold (2), hip and vertebral fractures 2.2- and 2.8-fold, respectively (≥7.5 mg daily) (3), and infections 1.7-fold (5-10 mg daily) (4). In the ADVOCATE trial in AAV, the avacopan group was superior to the prednisone group in sustaining remission at 52 weeks (5). The aim of the current analysis was to evaluate the indications for GC use in ADVOCATE and the association of its use with GC-related toxicity.
Methods: ADVOCATE was a Phase 3 randomized, double-blind, double-dummy trial comparing avacopan (replacing a standard oral prednisone taper) to a standard oral prednisone taper in 330 patients with AAV who also received rituximab or cyclophosphamide/azathioprine. All GC use and the reason for its use were recorded. EULAR-defined terms for adverse events potentially associated with GC use (GC-AEs) (6), were selected prior to database lock. Treatment group comparisons are presented here.
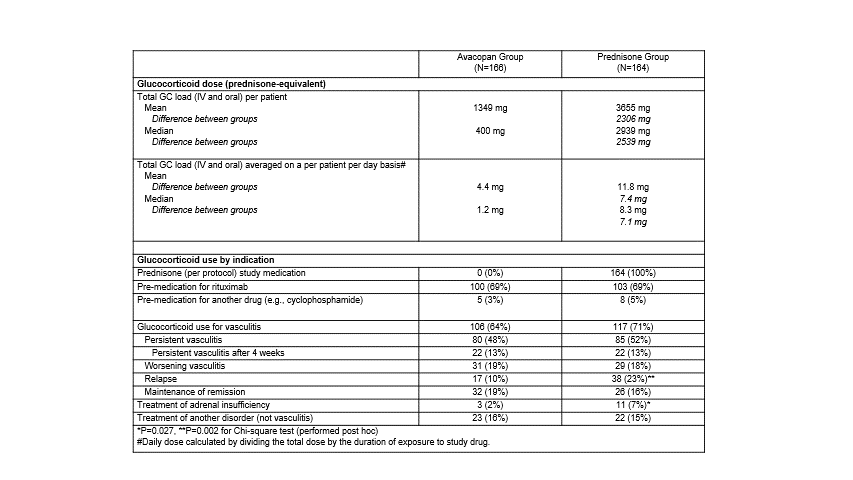
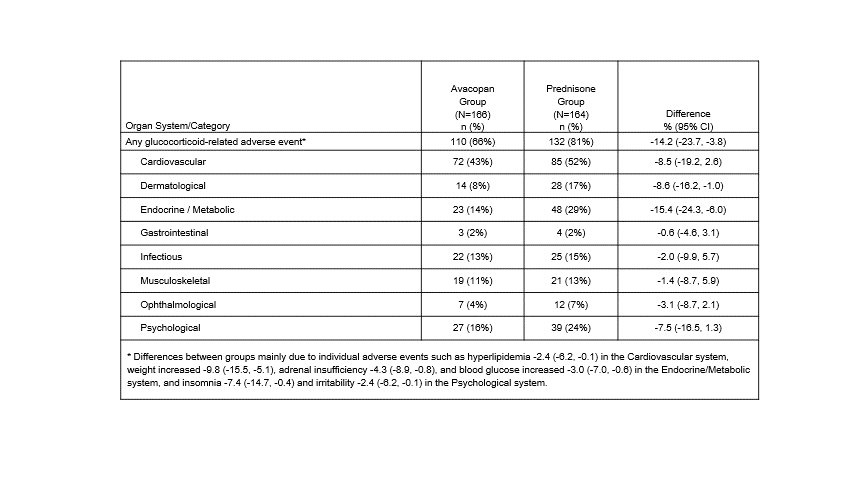
Results: With avacopan, the mean and median all-source GC load was 63% and 86% lower, respectively, compared to the prednisone group (Table 1; with the mean total GC and average daily dose 2306 mg lower and 7.4 mg lower, respectively). Prednisone study medication (for the standard oral taper) was only given to the prednisone group according to the study design. In the prednisone group, the incidence of GC use was 2.2-fold higher for relapse of vasculitis (P=0.002) and 3.7-fold higher for treatment of adrenal insufficiency (P=0.027). Statistically significantly, fewer patients in the avacopan group reported GC-AEs overall (66 vs 81%), endocrine/metabolic (14 vs 29%), and dermatologic (8 vs 17%) GC-AEs, and for every other category, the incidence of GC-AEs was numerically lower in the avacopan group (Table 2). The avacopan group had statistically fewer individual AEs of hyperlipidemia, increased weight, adrenal insufficiency, increased blood glucose, insomnia, and irritability.
Conclusion: In the ADVOCATE trial of avacopan for treatment of AAV, fewer patients taking avacopan reported AEs potentially associated with GC use. Also, fewer patients taking avacopan received GCs for treatment of relapses, consistent with lower risk of relapse observed in this group (5). These findings are consistent with previous studies, supporting the clinical significance of the lower total prednisone-equivalent doses in the avacopan group, both for reduction of GC toxicity within the first year, but also for the likely reduction of longer term impacts of GC use not captured in this study.
To cite this abstract in AMA style:
De Gomma E, Merkel P, George M, Rhee R, Kronbichler A, Jayne D, Yue H, Bekker P. Glucocorticoid Use and Related Adverse Events in ADVOCATE Trial of Avacopan in ANCA-Associated Vasculitis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/glucocorticoid-use-and-related-adverse-events-in-advocate-trial-of-avacopan-in-anca-associated-vasculitis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/glucocorticoid-use-and-related-adverse-events-in-advocate-trial-of-avacopan-in-anca-associated-vasculitis/