Session Information
Date: Sunday, November 8, 2020
Title: RA – Treatments Poster III: PROs, Biomarkers, Systemic Inflammation & Radiographs
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Glucocorticoid reduction in different profiles of patients with rheumatoid arthritis and sarilumab treatment. Real clinical practice studies are tasked with providing clinically relevant data that may clarify the benefits of this new IL-6 receptor blocking alternative, with higher affinity and dosing every two weeks. Beyond the magnitude of the effect that a drug can give us in a randomized, double-blind, control group clinical trial, the well-known selection biases of these studies do not always offer a representative picture of reality when using that drug on the day consultation day.
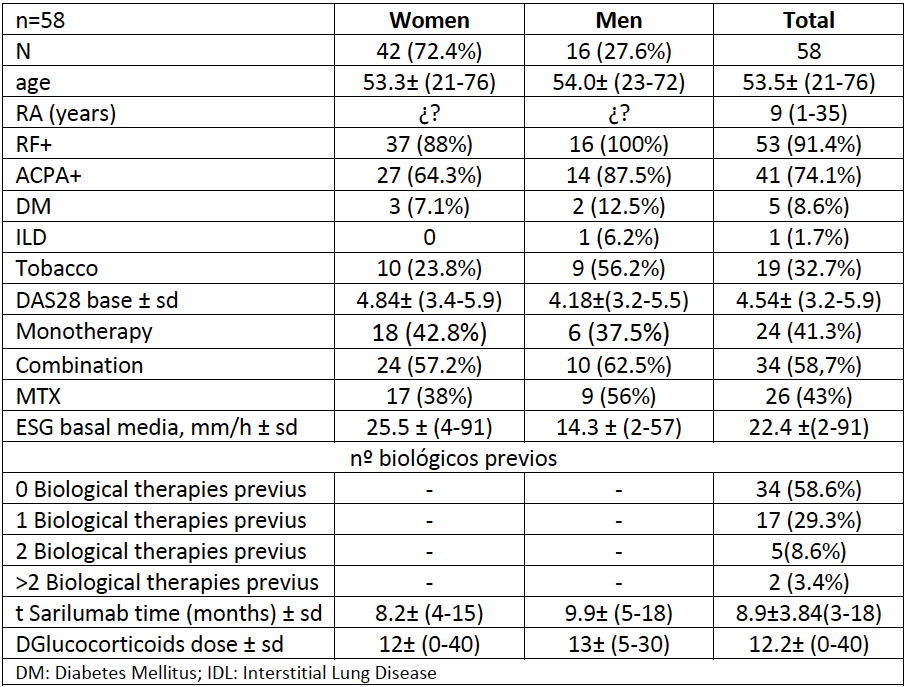
Methods: The data, hereafter commented, is the result of a retrospective analysis carried out in March 2020 on patients who were assigned to Sarilumab from September 2018 to November 2019 and therefore have at least a first clinical evaluation carried out 12 weeks after their Start. This study offers in our opinion several novelties: Firstly, almost two thirds of the analyzed cohort are patients naïve to another biological treatment (58.6%). This fact allows us to assess “the best opportunity” for a drug. We found it interesting to analyze two variables: the reduction of glucocorticoid doses secondary to the use of sarilumab and the efficacy depending on its association or not with a csDMARD.
A descriptive study was carried out; For the parametric distribution numerical variables, the mean and standard deviation are performed, reserving the median for those non-parametric distribution numerical variables. For qualitative variables, a study was carried out using frequency tables. And for the non-parametric ones, the Wilconxon test was used, which also show values of p < 0.05 For inferential studies, a 95% CI was used. All of them analyzed using SPSS 19.
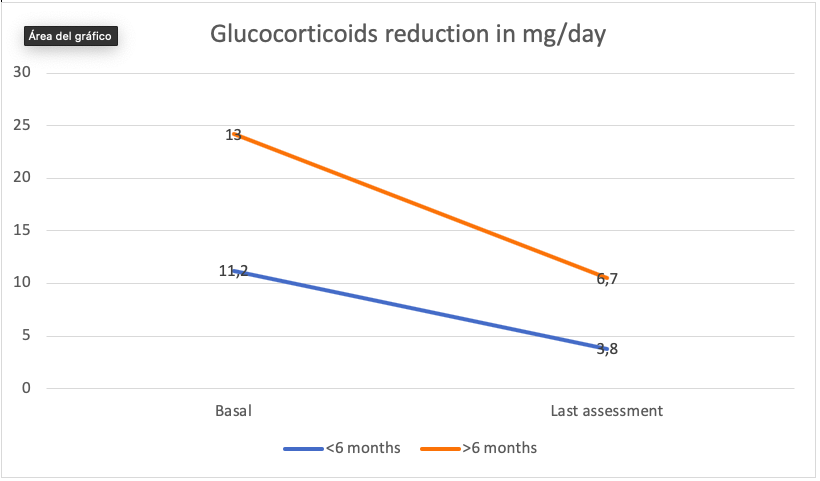
Results: The evolution in prednisone dose for the total of patients was striking and satisfactory. For a variable of temporary drug exposure, such as the one previously mentioned, we went from an average dose of 12mg / day to 5.2mg / day, a dose very close to the dose recommended in EULAR clinical practice guidelines, for the maintenance of a effective therapeutic strategy in an acceptable safety range. As in the case of efficacy assessed by an activity index, we stratified patients between those over 6 months exposed to Sarilumab and those with 6 or less months of treatment duration. In both cases the change had an effect size with clinical significance. In the population 6 months or less from the start of Sarilumab, the prednisone dose went from 11.2mg / day to mean prednisone 3.8mg / day and in patients with more than 6 months of treatment 12.9mg / day. at 6.7 mg / day.
Conclusion: In general, it could be stated that the drug Sarilumab presents in acceptable clinical practice an acceptable and predictable safety profile. From the point of view of the efficacy variable, we highlight one aspect: A good ability to reduce the steroid dose in a short time.
To cite this abstract in AMA style:
Rubio Romero E, Jiménez A, Martinez Perez R, Mendez Diaz L. Glucocorticoid Reduction in Different Profiles of Patients with Rheumatoid Arthritis and Treatment with Sarilumab [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/glucocorticoid-reduction-in-different-profiles-of-patients-with-rheumatoid-arthritis-and-treatment-with-sarilumab/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/glucocorticoid-reduction-in-different-profiles-of-patients-with-rheumatoid-arthritis-and-treatment-with-sarilumab/