Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Systemic lupus erythematosus (SLE) is a multi-system autoimmune disease associated with B cell hyperactivity driven by dysregulated type I IFN. Lyn-deficient mice develop lupus-like autoimmunity due to loss of inhibitory regulation of B cell receptor signalling, resulting in hyperactive B cells, excess IL-6 production, and tissue inflammation. Mice deficient in glucocorticoid-induced leucine zipper (GILZ), an intracellular protein involved in glucocorticoid actions, develop lupus-like autoimmunity and excess B cell activation. GILZ is suppressed in human SLE (Jones et al., 2016), but the effects of GILZ in murine models of SLE are unknown. We tested the hypothesis that loss of GILZ exacerbates autoimmunity in the Lyn-deficient murine model of lupus.
Methods: We crossed GILZ-deficient mice onto a Lyn-deficient background (GILZ-Lyn double knockout (DKO)) and compared them to WT and Lyn KO mice. The effects of GILZ deficiency on spleen weight, nephritis, serum autoantibodies and cytokines, and spleen cell expression of Type I interferon-induced genes (ISG) were examined.
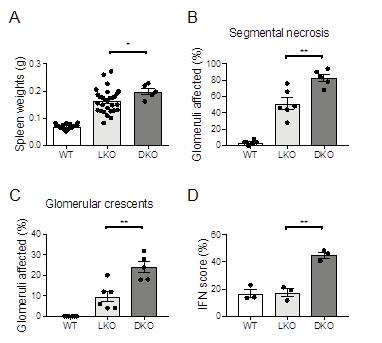
Results: We observed heightened lupus-like autoimmunity in GILZ-Lyn DKO mice, compared to Lyn KO, that include increased spleen weight (p=0.041) and more severe glomerulonephritis, including worse glomerular segmental necrosis (p=0.0051) and crescent formation (p=0.0044) (Fig 1A-C). Despite this, serum levels of serum autoantibodies against ENAs (dsDNA, histone, Sm, Jo-1, U1RNP, Ro52, ribosomes, and Scl-70) and a panel of serum pro-inflammatory cytokines (BAFF, IL-6, IFNγ, IL-10 and TNFα) measured by Luminex assay were unaffected. In contrast, GILZ deficiency in Lyn deficient mice resulted in increased expression of ISG (ifi44, usp18, oas3, isg15, mx1, and irf7) and an overall ISG signature (p=0.0023) (Fig 1D).
Conclusion: In Lyn KO lupus-prone mice, GILZ deficiency significantly exacerbated disease expression, accompanied by activation of Type I IFN, despite serum autoantibody titres and pro-inflammatory cytokines being unaffected. This suggests an inhibitory effect of endogenous GILZ on Type I IFN pathways and consequent tissue injury in this model, downstream of autoantibodies. A GILZ-based treatment could be a potential therapeutic strategy in SLE.
To cite this abstract in AMA style:
Nataraja C, Morand E, Lee J, Bennett T, Flynn J, Harris J, Jones S. Glucocorticoid-Induced Leucine Zipper (GILZ) Deficiency Worsens Autoimmunity in the Lyn-Deficient Murine Model of Lupus By Disinhibiting the Type I Interferon (IFN) Pathway [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/glucocorticoid-induced-leucine-zipper-gilz-deficiency-worsens-autoimmunity-in-the-lyn-deficient-murine-model-of-lupus-by-disinhibiting-the-type-i-interferon-ifn-pathway/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/glucocorticoid-induced-leucine-zipper-gilz-deficiency-worsens-autoimmunity-in-the-lyn-deficient-murine-model-of-lupus-by-disinhibiting-the-type-i-interferon-ifn-pathway/