Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Clinical trials of systemic lupus erythematosus (SLE) therapies have increased over the last decade, driven by evolving knowledge of targetable pathways. However, these trials are often small, leading to evidentiary gaps over which patients benefit from new therapies. Racially minoritized populations, which disproportionately experience severe disease, are underrepresented in published SLE trials, but little is known specifically about trials supporting drug development, including where enrollment sites are located and whether enrollment is representative of the US population with SLE. Our objective was to assess geographic and demographic enrollment patterns for registered SLE clinical trials.
Methods: Industry-sponsored clinical trials investigating small molecules and biologics for use in SLE or lupus nephritis with at least 1 US site were identified from ClinicalTrials.gov; cutaneous lupus trials were excluded. Study characteristics were abstracted from trial registrations. US sites were characterized at the county level using the National Center for Health Statistics Urban-Rural Classification Scheme and Health Resources and Services Administration Medically Underserved Area designation. For completed and terminated trials with results, participation-to-prevalence ratios (PPRs) were calculated by dividing proportions of participants by sex, ethnicity, and race by US population proportions across Centers for Disease Control and Prevention SLE registries; PPRs from 0.80 to 1.20 indicated adequate representation. Enrollment differences across study characteristics were evaluated using Kruskal-Wallis tests.
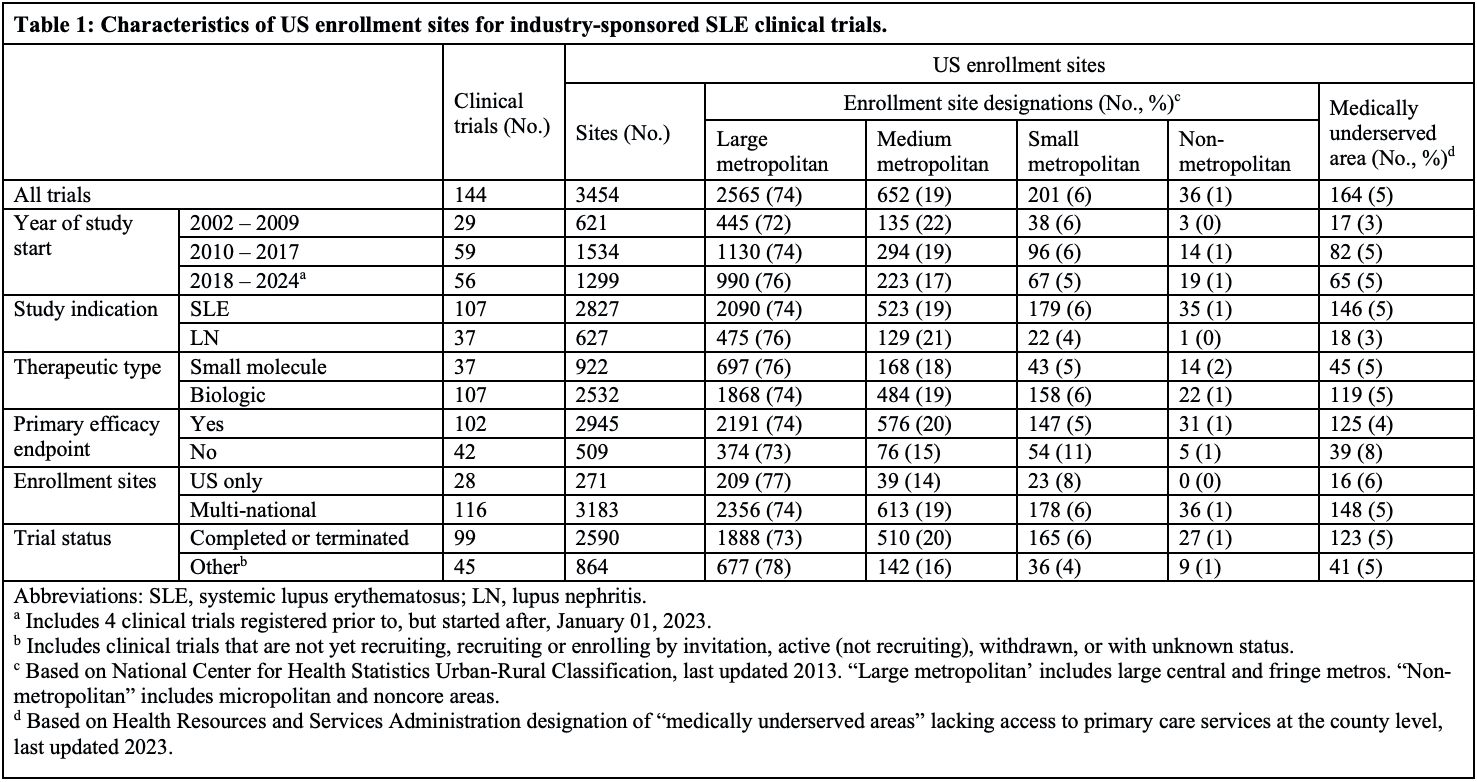
Results: Among 144 industry-sponsored SLE clinical trials, 3454 US sites were reported, including 2565 (74%) in large metropolitan counties and 237 (7%) in small- or non-metropolitan counties (Table 1). Only 164 sites (5%) were in medically underserved counties. Female participants were adequately represented relative to the US population with SLE (median PPR 1.03, IQR 0.99-1.05) in 81 trials reporting enrollment by sex (Table 2). Among 72 trials reporting enrollment for at least 1 race, representation was inadequate for Black (median PPR 0.38, IQR 0.25-0.62) and American Indian/Alaska Native participants (median PPR 0.22, IQR 0-1.14), and adequate for White participants (median PPR 1.04, IQR 0.76-1.18). Asian participants were overrepresented in trials overall (median PPR 2.08, IQR 0.59-3.74), but underrepresented in trials with US sites only. Among 47 trials reporting ethnicity data, Hispanic participants were adequately represented (median PPR 1.01, IQR 0.84-1.73).
Conclusion: Among registered industry-sponsored clinical trials of SLE therapies, US enrollment sites were concentrated in metropolitan areas; only 5% were in medically underserved counties. Female, White, Asian, and Hispanic participants were adequately represented in trials overall, but Black and American Indian/Alaska Native participants were underrepresented relative to the US population with SLE. Future analyses are needed to further characterize regional enrollment, and to assess the alignment of research practices with the needs of clinicians seeking new therapies for SLE.
To cite this abstract in AMA style:
Skydel J, Ramachandran R, Suttiratana S, Ross J, Wallach J, Burns C. Geographic and Demographic Representation in Industry-sponsored, US-based Clinical Trials of Systemic Lupus Erythematosus Therapies [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/geographic-and-demographic-representation-in-industry-sponsored-us-based-clinical-trials-of-systemic-lupus-erythematosus-therapies/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/geographic-and-demographic-representation-in-industry-sponsored-us-based-clinical-trials-of-systemic-lupus-erythematosus-therapies/