Session Information
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: Teratogenic disease-modifying anti-rheumatic disease modifying drugs (DMARDs) are often prescribed to women of childbearing age. Contraception represents an important area of reproductive health for female patients with rheumatic diseases. Counseling prior to starting DMARDs can help prevent the potential pregnancy risks associated with such medications. Methotrexate (MTX), cyclophosphamide (CP), leflunomide (LFN) and mycophenolate (MMF) are commonly used DMARDs, which can be highly teratogenic.
Methods: The use of high-risk medications was retrospectively evaluated in 484 female rheumatic disease patients of childbearing age (13 to 45 years old) as documented in the electronic medical records of Upstate University Hospital between 2013-2019. Charts were reviewed for medication use, documentation of education and use birth control (medication, sterilization, or postmenopausal) (Table 1 &2). Statistical analysis was performed with chi-square or Fisher’s exact test using GraphPad version 8.0 software. Two-tailed p < 0.05 was considered significant.
Results: 484 DMARD-treated patients were evaluated for documentation of education on drug teratogenicity and compliance with use of birth controls. 76% of patients were Caucasian, 18% were African American or Hispanic and 6% were Indian, Asian or Native American. 50% (243/484) of patients were educated regarding potential teratogenic complications that only resulted in greater use of contraceptives in methotrexate-treated patients with rheumatoid arthritis (Table 1).
Conclusion: In the USA, it is estimated that about 5.8% of all pregnancies are exposed to category D or X medications [1]. More than 50% of all pregnancies in the USA are unplanned [2]. This is concerning, especially for our rheumatic disease patients who are already at risk. Potential teratogenic medications should be thoroughly reviewed with patients so that preventive measures could be employed. Despite the common consensus for contraceptive education, there still remains a gap in implementing this standard clinical practice, especially among patients with SLE [3,4]. In our facility, teratogenicity of potential drugs and family counseling was provided only 50% of the time, indicating major gaps in family planning counseling among childbearing aged women with rheumatic diseases. To ensure that our patients receive proper family planning and counseling, a collaboration among rheumatologist, primary care physicians and obstetrician-gynecologists is critical. Our study demonstrates critical gaps in counseling and compliance with use of teratogenic medications. Future intervention should address barriers in counseling, compliance and documentation of teratogenic medication use.
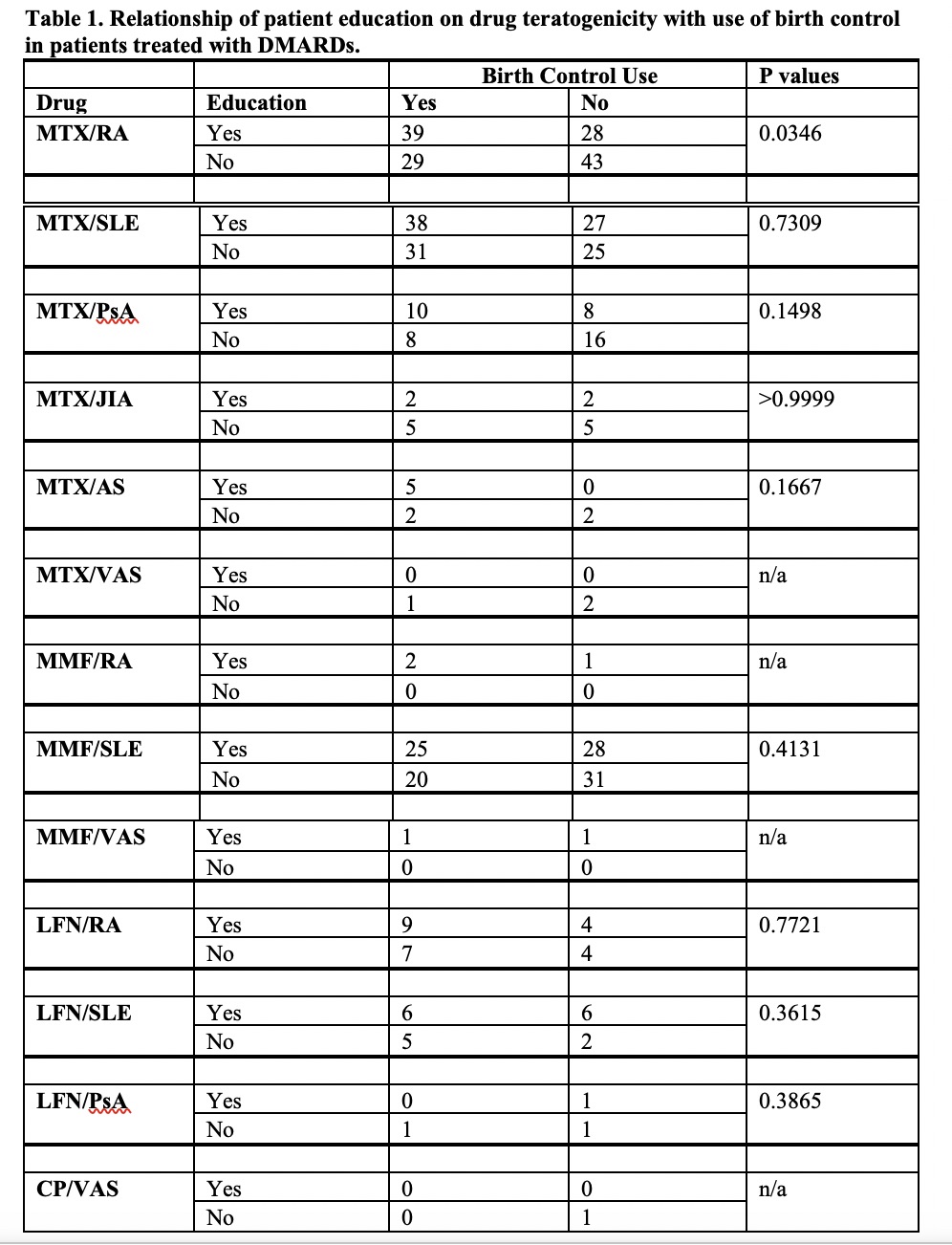 SLE, systemic lupus erythematosus; RA, rheumatoid arthritis; PsA, psoriatic arthritis; JIA, juvenile inflammatory arthritis; AS, ankylosing spondylitis; VAS, vasculitis. MTX, methotrexate, MMF, mycophenolate mofetic or mycophenolic acid; LFN, leflunomide; CP, cyclophosphamide; edu, educated; BC, birth control.
SLE, systemic lupus erythematosus; RA, rheumatoid arthritis; PsA, psoriatic arthritis; JIA, juvenile inflammatory arthritis; AS, ankylosing spondylitis; VAS, vasculitis. MTX, methotrexate, MMF, mycophenolate mofetic or mycophenolic acid; LFN, leflunomide; CP, cyclophosphamide; edu, educated; BC, birth control.
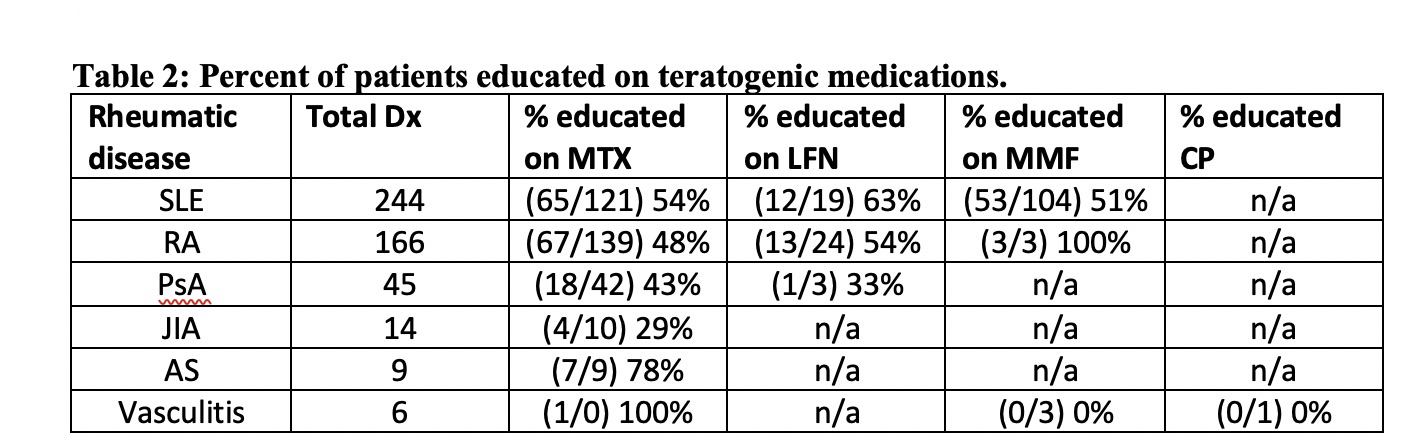 SLE, systemic lupus erythematosus; RA, rheumatoid arthritis; PsA, psoriatic arthritis; JIA, juvenile inflammatory arthritis; AS, ankylosing spondylitis. MTX, methotrexate, MMF, mycophenolate mofetic or mycophenolic acid; LFN, leflunomide; CP, cyclophosphamide; edu, educated; BC, birth control.
SLE, systemic lupus erythematosus; RA, rheumatoid arthritis; PsA, psoriatic arthritis; JIA, juvenile inflammatory arthritis; AS, ankylosing spondylitis. MTX, methotrexate, MMF, mycophenolate mofetic or mycophenolic acid; LFN, leflunomide; CP, cyclophosphamide; edu, educated; BC, birth control.
To cite this abstract in AMA style:
Perl A, Mian S, Ben Gabr J. Gap in Contraceptive Education to Females with Rheumatic Disease on Teratogenic Medications [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/gap-in-contraceptive-education-to-females-with-rheumatic-disease-on-teratogenic-medications/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/gap-in-contraceptive-education-to-females-with-rheumatic-disease-on-teratogenic-medications/
