Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Systemic sclerosis (SSc) can greatly impact the patients’ quality of life due to its multisystem manifestations. The health assessment questionnaire (HAQ) is one of the most commonly used measures of disability in musculoskeletal disorders and was extended to form the scleroderma HAQ (SHAQ), a more disease-specific disability scale that incorporates the HAQ and 5 visual analogue scales (VAS) into one score. This cross-sectional study aims to identify contributors of disability in SSc by means of the SHAQ.
Methods: Adult patients from the prospective DeSScipher cohort were included in the analysis if they had one complete SHAQ recorded (range 0-3) and fulfilled either the 1980 ACR or the 2013 ACR/EULAR criteria for SSc. Multiple linear regression analysis was used to assess the combined effect of factors defined a priori and possibly associated with a lower SHAQ.
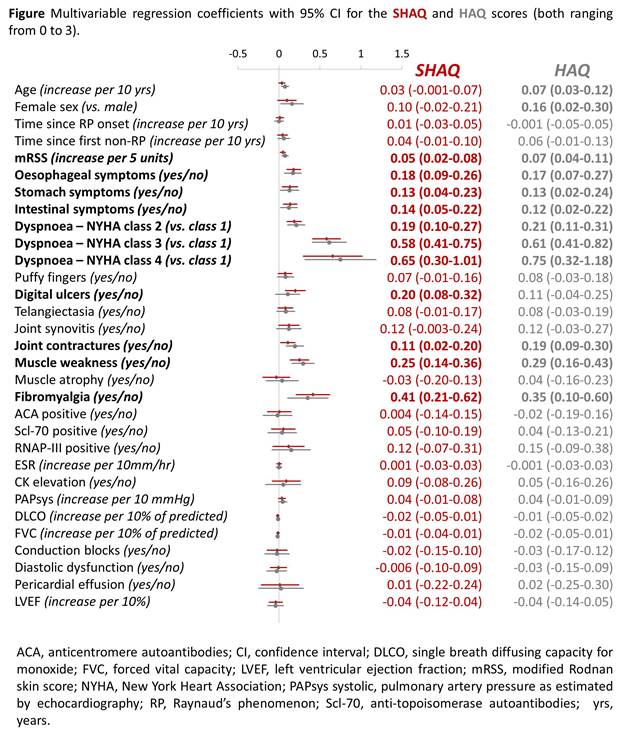
Results: Between June 2013 and January 2016, 813 patients had one complete SHAQ recorded (34% of all patients followed in the DeSScipher cohort). The mean SHAQ score was 0.86 (standard deviation [SD] 0.65) and the mean HAQ score was 0.92 (SD 0.77). 60% of patients were in the “mild to moderate difficulty” SHAQ category (score of 0-1), 34% in the “moderate to severe disability” category (score of 1-2) and 6% in the “severe to very severe disability” category (score of 2-3). In order of magnitude, the means of the five VASs included in the SHAQ were: Overall disease severity (30, IQR 10-51), Raynaud’s phenomenon (21, IQR 3-50), pulmonary symptoms (10, IQR 1-40), gastrointestinal symptoms (6, IQR 1-31) and digital ulcers (2, IQR 1-30). In multiple linear regression, the main contributor to functional disability was dyspnea. The SHAQ scores reported by patients with NYHA class 4, 3 or 2 were on average 0.65 units (95% confidence interval [CI] 0.30-1.01), 0.58 units (95%CI 0.41-0.75) and 0.19 units (95%CI 0.10-0.27) higher than that of patients with NYHA class 1. The presence of fibromyalgia (0.41 units, 95%CI 0.21-0.62) as well as muscle weakness (0.25 units, 95%CI 0.14-0.36) were also associated with higher levels of disability (Figure). Patients reporting esophageal, gastric and intestinal symptoms simultaneously had, on average, a SHAQ score of 0.45 units (95% CI 0.33-0.58) higher than patients reporting no gastrointestinal symptoms. Patients with symptoms in two gastrointestinal regions had a SHAQ score of 0.28 units (95%CI 0.17-0.39) and in one region of 0.13 units (95% CI 0.04-0.23) higher than patients with no symptoms. The contributing factors to an impaired functional ability were similar in patients with diffuse SSc and patients with limited SSc.
Conclusion: Patients perceive dyspnea, pain, muscle weakness and gastrointestinal symptoms as the main factors driving their level of disability. Acknowledgement: The DeSScipher project was funded by the European Community’s Framework Programme 7 under grant agreement N° 305495.
To cite this abstract in AMA style:
Jaeger VK, Czirjak L, Lóránd V, Valentini G, Vettori S, Del Galdo F, Abignano G, Distler O, Maurer B, Denton C, Nihtyanova S, Allanore Y, Avouac J, Riemekasten G, Siegert E, Huscher D, Matucci-Cerinic M, Guiducci S, Frerix M, Tarner IH, Garay-Toth B, Ananieva LP, Cozzi F, Yavuz S, Hunzelmann N, Vacca A, Schmeiser T, Rednic S, Riccieri V, Krummel-Lorenz B, Gabrielli A, Garcia De La Peña P, Ancuta C, Müller-Ladner U, Walker UA. Functional Disability and Its Predictors in Systemic Sclerosis: A Study from the Desscipher Project within the European Scleroderma Trials and Research Group [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/functional-disability-and-its-predictors-in-systemic-sclerosis-a-study-from-the-desscipher-project-within-the-european-scleroderma-trials-and-research-group/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/functional-disability-and-its-predictors-in-systemic-sclerosis-a-study-from-the-desscipher-project-within-the-european-scleroderma-trials-and-research-group/