Session Information
Date: Sunday, November 12, 2023
Title: (0325–0344) Patient Outcomes, Preferences, & Attitudes Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Post-viral fibromyalgia is a chronic condition that can develop in individuals following a viral infection, such as COVID-19. Recent studies have shown that approximately 30% of post-COVID19 patients satisfy the ACR criteria for fibromyalgia, experiencing symptoms such as chronic fatigue, widespread pain, sleep impairment, anxiety and depression.
Digital health interventions have demonstrated efficacy by providing disease monitoring, management and multimodal interventions to chronic pain patients. However, the widespread adoption and adherence to these interventions remain an obstacle. To address these challenges, this study aimed to develop a patient-centered digital health management app tailored specifically for post-viral fibromyalgia patients. By incorporating patient preferences through surveys and conducting usability testing, the study sought to enhance the usability of the app and the engagement of patients. Consequently, improving self-management, leading to improved patient outcomes and a better quality of life.
Methods: This study employed an explanatory design. Patient preference surveys were conducted among individuals recruited from “Long-COVID Schweiz”, a post-COVID19 patients association. Usability testing was run with post-viral fibromyalgia patients that were enrolled in the multimodal care program for chronic pain management at the University Hospital of Lausanne (Switzerland).
Data collected from surveys consisted of qualitative preference data related to functional design, interactions with the app, patient reported outcomes, type of monitored data and expected content. Usability testing data under the form of notes, interviews and patients’ feedback was collected by observing patients using the newly developed prototype.
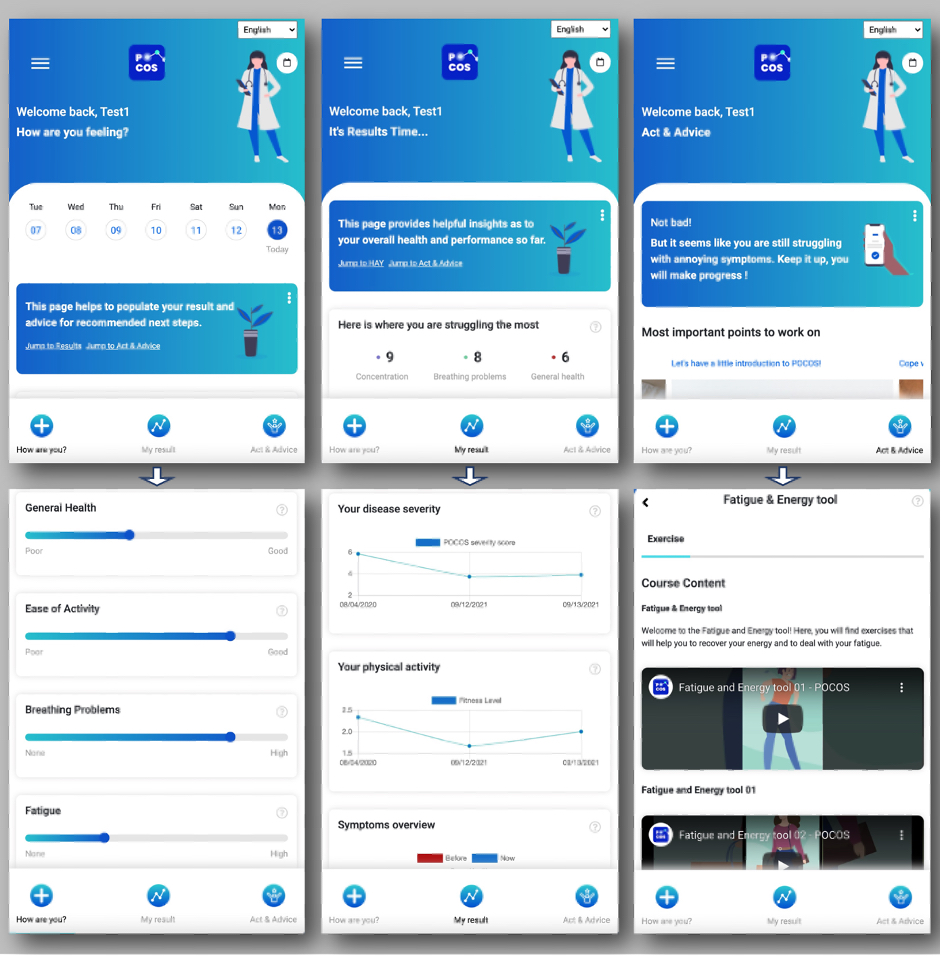
Results: The frontend design of the app is shown in Figure 1. Among the 53 patients who responded to patient preference surveys, 90% preferred a regular symptoms list questionnaire over a chatbot to collect patient reported outcomes. Longitudinal symptom evaluation is shown in the “my result” section. 81% of patients expressed their wish that their symptoms are displayed with a benchmark of all other patients and to learn what has helped other patients with similar symptoms. A majority of them were also interested in links to patient communities (63%). Therefore, an anonymized discussion forum has been added. Patients preferred active training programs (63%) and information (59%) over interactive and gamified content (15%).
Among the 6 patients who participated in the usability testing, only 2 showed sufficient understanding of the functionalities and managed to navigate through the app without additional help, revealing the importance of the onboarding process.
Conclusion: Patient preference surveys guided the development of a patient-centric digital health solution, while usability testing identified issues with the onboarding process, requiring further study to investigate the impact of the onboarding on patient adoption and ultimately enhance engagement.
To cite this abstract in AMA style:
Blanchard M, Ming Azevedo P, Prétat T, Koller C, Hügle T. From Patient Needs to Platform Design: Using Patient Preference to Guide the Development of a Post-Viral Fibromyalgia Management App [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/from-patient-needs-to-platform-design-using-patient-preference-to-guide-the-development-of-a-post-viral-fibromyalgia-management-app/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/from-patient-needs-to-platform-design-using-patient-preference-to-guide-the-development-of-a-post-viral-fibromyalgia-management-app/