Session Information
Date: Saturday, November 7, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Guselkumab (GUS), a fully human monoclonal antibody, selectively binds and blocks IL-23. VOYAGE 1 and VOYAGE 2 are two ongoing Phase 3, randomized, double-blind, placebo (PBO)/active comparator-controlled clinical trials of GUS in patients with moderate-to-severe psoriasis (PsO). This post-hoc analysis reported pooled results through 4 years among a subgroup of moderate-to-severe PsO patients with self-reported PsA at baseline.
Methods: 1829 patients were randomized to GUS 100 mg at Weeks 0, 4, and 12, then every 8 weeks (q8wk); PBO at Weeks 0, 4, and 12, GUS at Weeks 16 and 20 then q8wk; or adalimumab (ADA) 80 mg at Week 0, 40 mg at Week 1, then 40 mg q2wk until Week 47 (VOYAGE 1) or Week 23 (VOYAGE 2). In VOYAGE 1, all patients received open-label GUS 100 mg q8wk during Weeks 52-204. VOYAGE 2 incorporated a randomized withdrawal study design, followed by open-label GUS during Weeks 76-204. Pooled subgroup analyses using the combined GUS group were conducted based on self-reported PsA status at baseline. Efficacy based on Investigator Global Assessment (IGA) score and Psoriasis Area and Severity Index (PASI) response was assessed using prespecified treatment failure rules (nonresponder status for all time points after discontinuing due to lack of efficacy, worsening of PsO, or use of a prohibited treatment).
Results: For pooled VOYAGE 1 and VOYAGE 2 patients (N=1721), combined GUS and ADA→GUS response rates at Weeks 100, 156, and 204 were: PASI 90 80.6%, 80.0%, and 80.4%; PASI 100 50.1%, 49.9%, and 52.2%; IGA 0/1 83.6%, 83.3%, and 81.7%; and IGA 0 54.3%, 52.9%, and 53.9, respectively. In the pooled subgroup analysis of patients with and without PsA, response rates were similar across the Week 100, Week 156, and Week 204 evaluations (Table). Rates of adverse events through Week 204 were comparable for patients with PsA vs those without PsA at baseline.
Conclusion: Among GUS-treated patients with moderate-to-severe PsO with and without self-reported PsA at baseline, stable, durable, and high levels of skin responses, as well as comparable safety outcomes, through 4 years were observed.
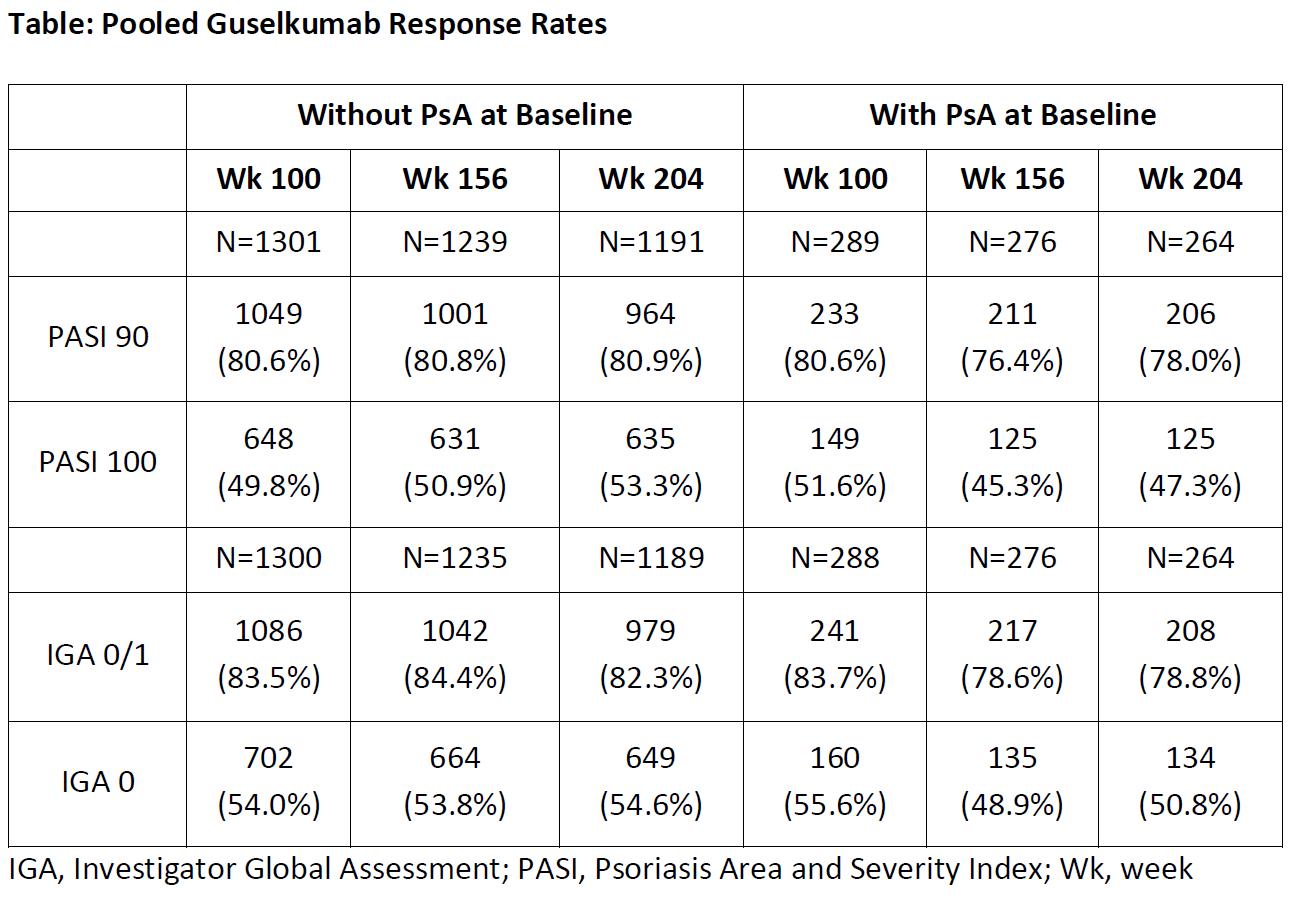 Table: Pooled Guselkumab Response Rates
Table: Pooled Guselkumab Response Rates
To cite this abstract in AMA style:
Reich K, Dutz J, Foley P, Thaçi D, Vender R, Song M, Miller M, Yu Y, Li S, Shen Y, Armstrong A. Four-Year Efficacy and Safety of Guselkumab in Psoriasis Patients with and Without Psoriatic Arthritis: A Pooled Analysis from VOYAGE 1 and VOYAGE 2 [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/four-year-efficacy-and-safety-of-guselkumab-in-psoriasis-patients-with-and-without-psoriatic-arthritis-a-pooled-analysis-from-voyage-1-and-voyage-2/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/four-year-efficacy-and-safety-of-guselkumab-in-psoriasis-patients-with-and-without-psoriatic-arthritis-a-pooled-analysis-from-voyage-1-and-voyage-2/
