Session Information
Date: Tuesday, November 12, 2019
Title: Systemic Sclerosis & Related Disorders – Clinical Poster III
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Interstitial Lung Disease (ILD) is the leading cause of morbidity and mortality in systemic sclerosis (SSc) patients. Forced Vital Capacity (FVC, recorded as % predicted) is a valid outcome measure to assess lung function in patients with ILD. Our objective was to determine whether varying trajectories of FVC in patients with SSc-ILD were associated with different phenotypes of disease.
Methods: Patient data was collected from the electronic medical records of SSc patients seen in the senior author’s clinic. SSc patients who completed Chest Computed Tomography (CTs), to evaluate presence of ILD, and had at least 3 FVC measurements that were at least 3 months apart were included in the analysis. A latent class growth analysis model, PROC TRAJ, was used to identify heterogeneous groups among SSc-ILD patients according to their FVC trajectories over time.T-test and Chi-square test were used to compare baseline characteristics among the identified subpopulations.
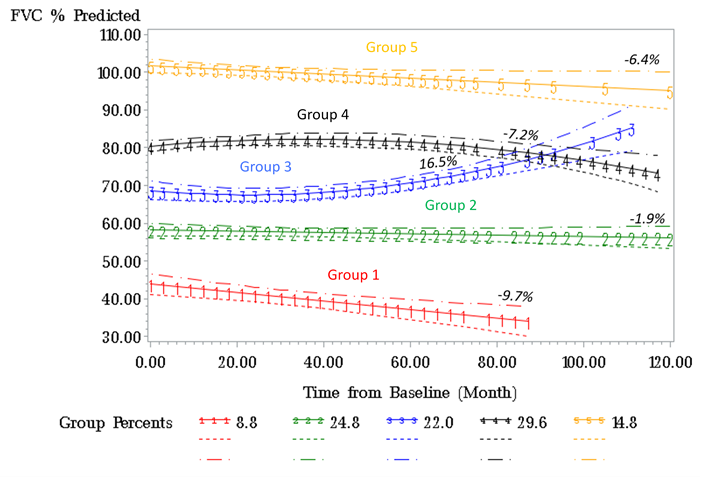
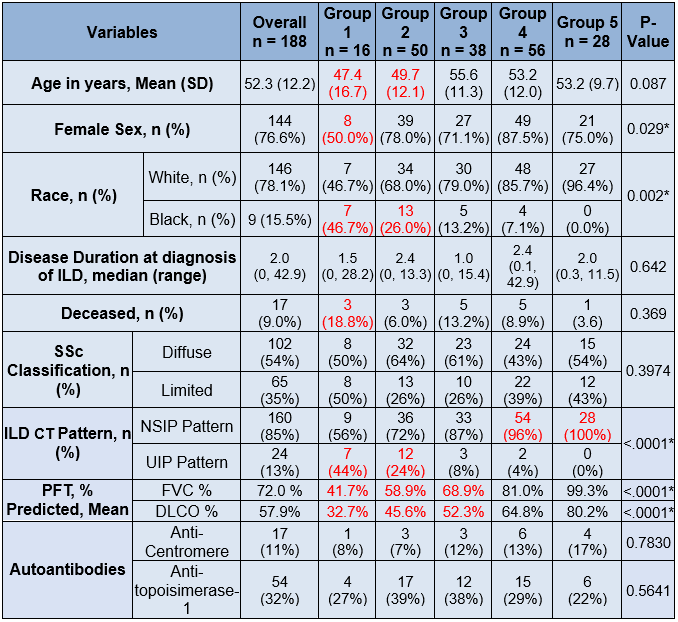
Results: Out of 484 SSc patients in our cohort, 293 had evidence of ILD on a chest CT of which 86% of which were high resolution CTs (HRCTS). Of the 293 patients with ILD,188 also had at least 3 FVC measurements performed at least 3 months apart. The median [IQR] for follow-up was 42 [25] months and estimated change in FVC among all patients was –2.87% in 100 months. We identified 5 subpopulations with distinct trajectories of FVC% in SSc-ILD patients (group mean trajectories as shown in Figure 1). Groups 1-3 had abnormal baseline FVC% (mean < 80%) whereas Groups 4 and 5 had normal mean baseline FVC% (Table 1). The majority of our cohort fell into Group 2 (27%), Group 3 (22%), or Group 4 (30%). Group 1 had the lowest estimated FVC% at baseline and greatest decline in estimated FVC%, younger age, higher prevalence of males and African-Americans, and had higher deaths compared to the other groups; the median disease duration was similar in the 5 groups (Table 1) at baseline. Significant differences were found in the patterns of ILD on chest CTs, with Usual Interstitial Pneumonitis (UIP) being more prevalent in low FVC Groups (1 and 2).
Conclusion: Our results suggest 5 distinct groups of patients with SSc-ILD with respect to change in FVC over time. Ongoing work will quantify degree of ILD on chest CTs and the impact of immunosuppressive therapy on long term outcomes in this cohort.
To cite this abstract in AMA style:
Habib M, Jaafar S, Park A, Huang S, Ye W, Nagaraja V, White E, Flaherty K, Khanna D. Forced Vital Capacity Trajectories for Systemic Sclerosis-associated Interstitial Lung Disease—Analysis from the University of Michigan Scleroderma Cohort [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/forced-vital-capacity-trajectories-for-systemic-sclerosis-associated-interstitial-lung-disease-analysis-from-the-university-of-michigan-scleroderma-cohort/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/forced-vital-capacity-trajectories-for-systemic-sclerosis-associated-interstitial-lung-disease-analysis-from-the-university-of-michigan-scleroderma-cohort/