Session Information
Date: Saturday, November 6, 2021
Title: Epidemiology & Public Health Poster I: COVID-19 & Vaccination (0084–0117)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: Although persons with immune mediated inflammatory diseases (IMIDs) are at increased risk of herpes zoster (HZ), there are limited data published on the safety of recombinant zoster vaccine (RZV, Shingrix), or novel vaccine adjuvants, in these populations. We conducted a self-controlled case series analysis (SCCS) to evaluate the risk of possible vaccine-related flares following RZV vaccination in adults with IMIDs.
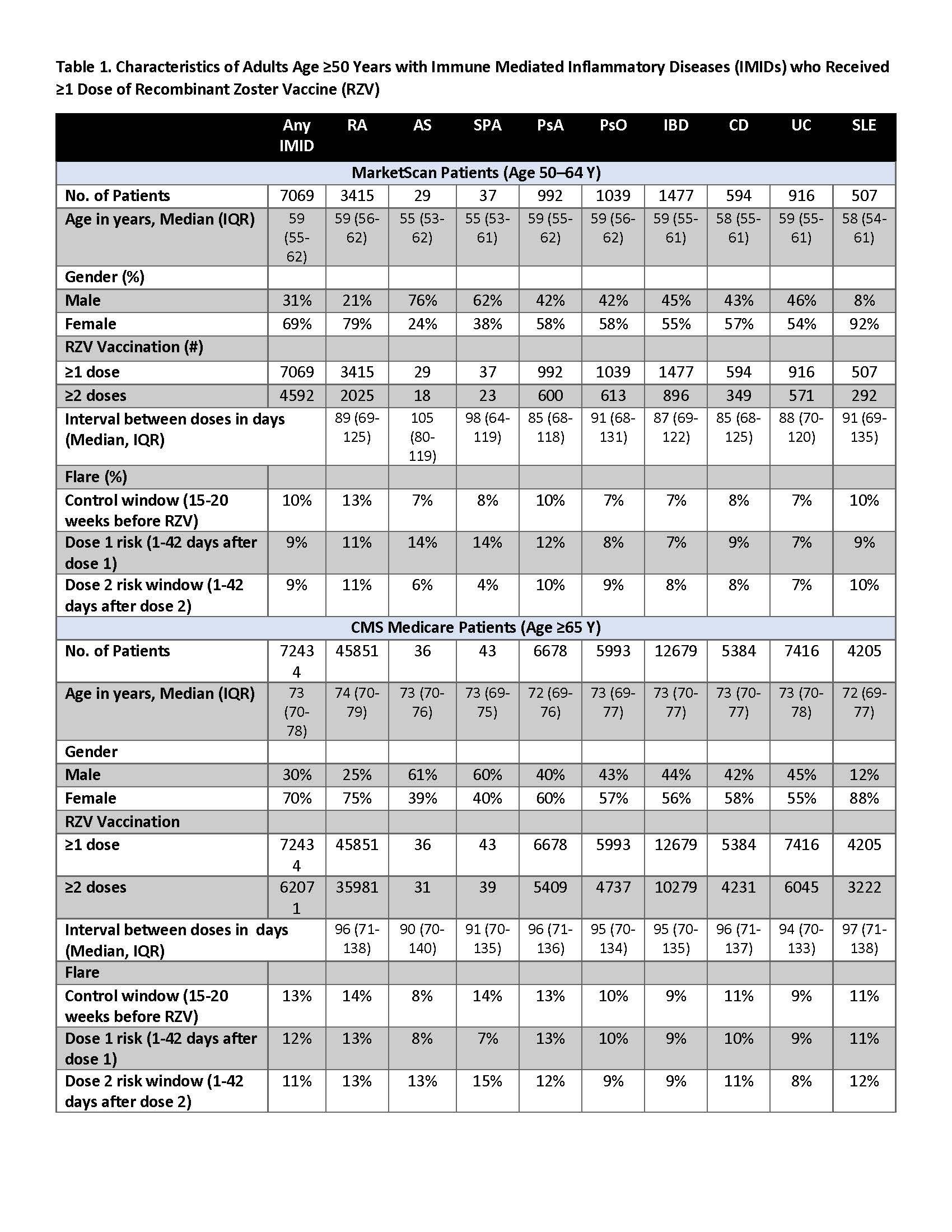
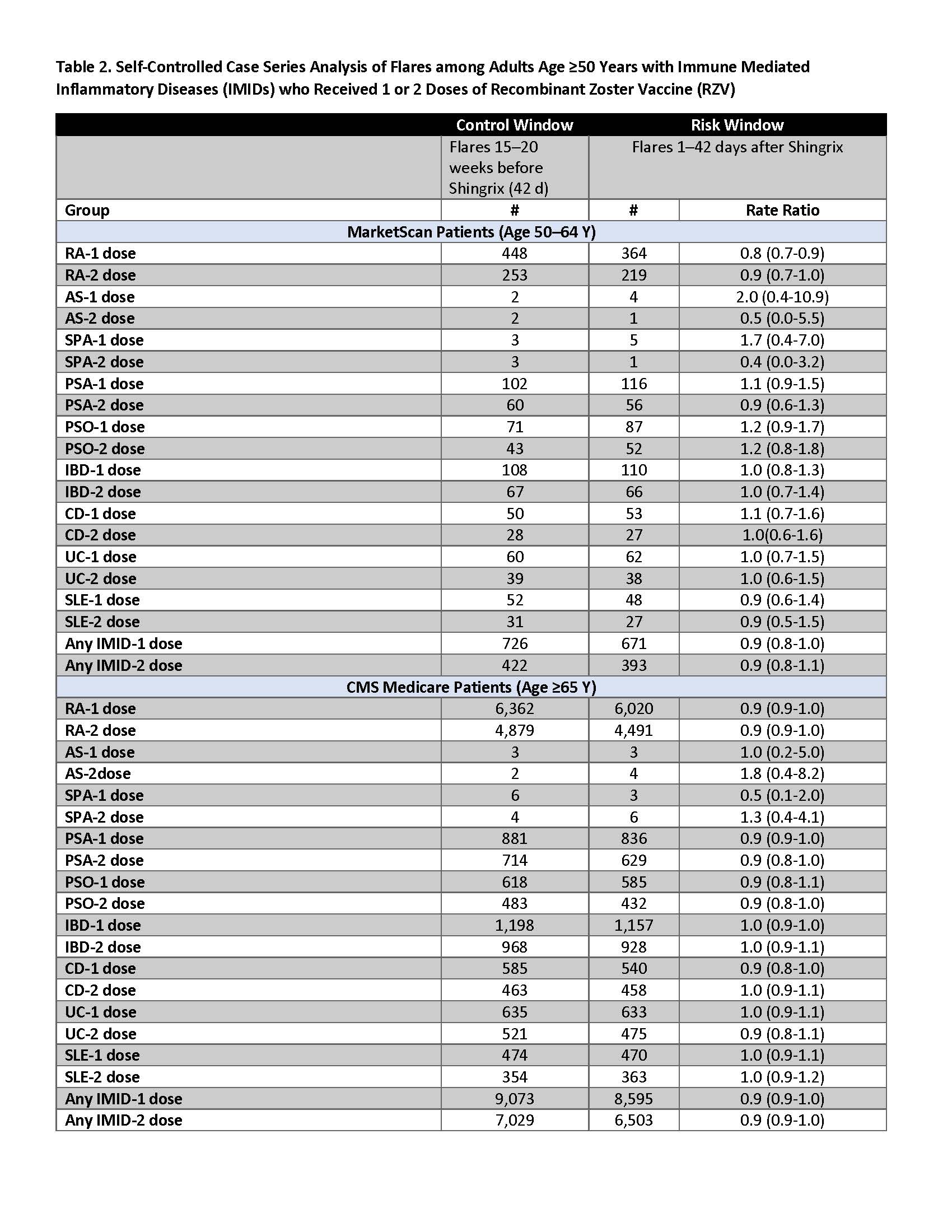
Methods: We used medical claims data from the 2017–2019 IBM® MarketScan® (persons age 50–64 years) and 2017–2020 Centers for Medicare and Medicaid Services Medicare (persons age ≥65 years) databases to examine flares after RZV vaccination in persons with IMIDs (Rheumatoid Arthritis, RA; Ankylosing Spondylitis, AS; Axial Spondyloarthritis, SpA; Psoriatic Arthritis, PsA; Psoriasis, PsO; Inflammatory Bowel Disease, IBD; Crohn’s Disease, CD; Ulcerative Colitis, UD; Systemic lupus erythematosus, SLE). IMIDs were defined using all 3 of the following criteria: (a) ≥2 outpatient visits for their respective conditions, and (b) ≥1 claim for disease-specific medications, and (c) ≥1 visit to a relevant specialist (rheumatologist for RA, AS, SpA, PsA, SLE; gastroenterologist for IBD, CD, UC; dermatologist for PsO). RZV vaccination was defined using NDC and CPT codes. Presumed flares were defined as hospitalization or ER visit for their condition, or treatment with a Medrol Dosepak or steroid injection. We used SCCS methods to compare the rates of flares 1–42 days after (risk window) as compared to 98–140 days prior to RZV vaccination (control window) within individuals.
Results: There were 7,069 50–64-year-olds and 72,434 ≥65-year-olds with both an IMID and ≥1 dose of RZV [Table 1]. Among 50–64-year-olds, 10% developed flares during the control window, and 9% developed flares in the risk window following 1 or 2 doses of RZV. Among ≥65-year-olds, 13% developed flares during the control window, and 11–12% developed flares in the risk window following 1 or 2 doses of RZV. We did not find a statistically significant increase in flares following RZV vaccination for any IMID condition in either age group following either 1 or 2-doses of RZV [Table 2].
Conclusion: This observational study provides new data on the safety of RZV vaccination in adults age ≥50 years with selected IMIDs. Within the limitations of administrative data and our presumed flare definition, we did not find a significant increase in presumed flares for any IMID condition evaluated or by RZV vaccine dose. There were a number of limitations with this study related to distinguishing vaccine-induced flares from reactogenicity and disease worsening. Additional work is ongoing to refine and validate IMID case and flare definitions. Further evaluation of the safety of RZV vaccination in this population is needed to inform vaccine policy recommendations and clinical guidance.
To cite this abstract in AMA style:
Curtis J, Su y, Xie F, Clinton c. Flares Following Herpes Zoster Recombinant Zoster Vaccination Among Adults Age ≥50 Years with Immune Mediated Inflammatory Diseases in the United States [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/flares-following-herpes-zoster-recombinant-zoster-vaccination-among-adults-age-%e2%89%a550-years-with-immune-mediated-inflammatory-diseases-in-the-united-states/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/flares-following-herpes-zoster-recombinant-zoster-vaccination-among-adults-age-%e2%89%a550-years-with-immune-mediated-inflammatory-diseases-in-the-united-states/