Session Information
Date: Sunday, November 7, 2021
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Interleukin-6 (IL-6) plays a key role in inflammatory and immune responses. Tocilizumab (TCZ) is a recombinant humanized monoclonal antibody of the IL-6 receptor which inhibits IL-6-mediated signaling. TCZ is available as an intravenous (IV) formulation (TCZ-IV) and a subcutaneous (SC) formulation (TCZ-SC). The present study aimed to evaluate the incidence and risk factors for RA flares after switching to TCZ-SC in stable RA patients as TCZ-IV.
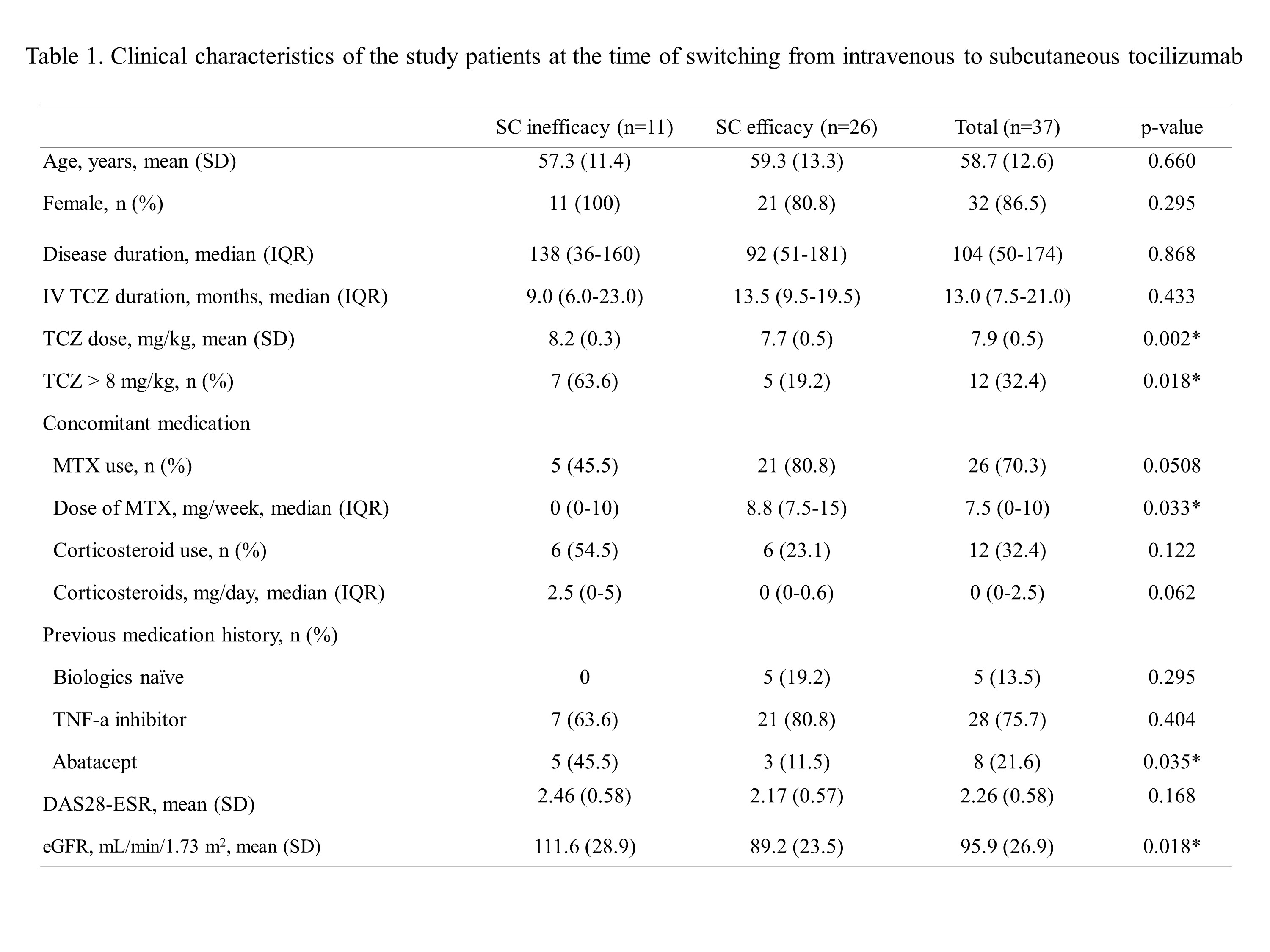
Methods: We retrospectively evaluated the incidence of RA flares in patients who switched from TCZ-IV to TCZ-SC in single tertiary hospital between January 2013 and April 2020. Patients were divided into two groups based on RA disease activity as assessed by Disease Activity Score in 28 joints (DAS28) after switching from TCZ-IV to TCZ-SC (“SC inefficacy group” versus “SC efficacy group”). Factors associated with RA flares were evaluated by multivariate analysis.
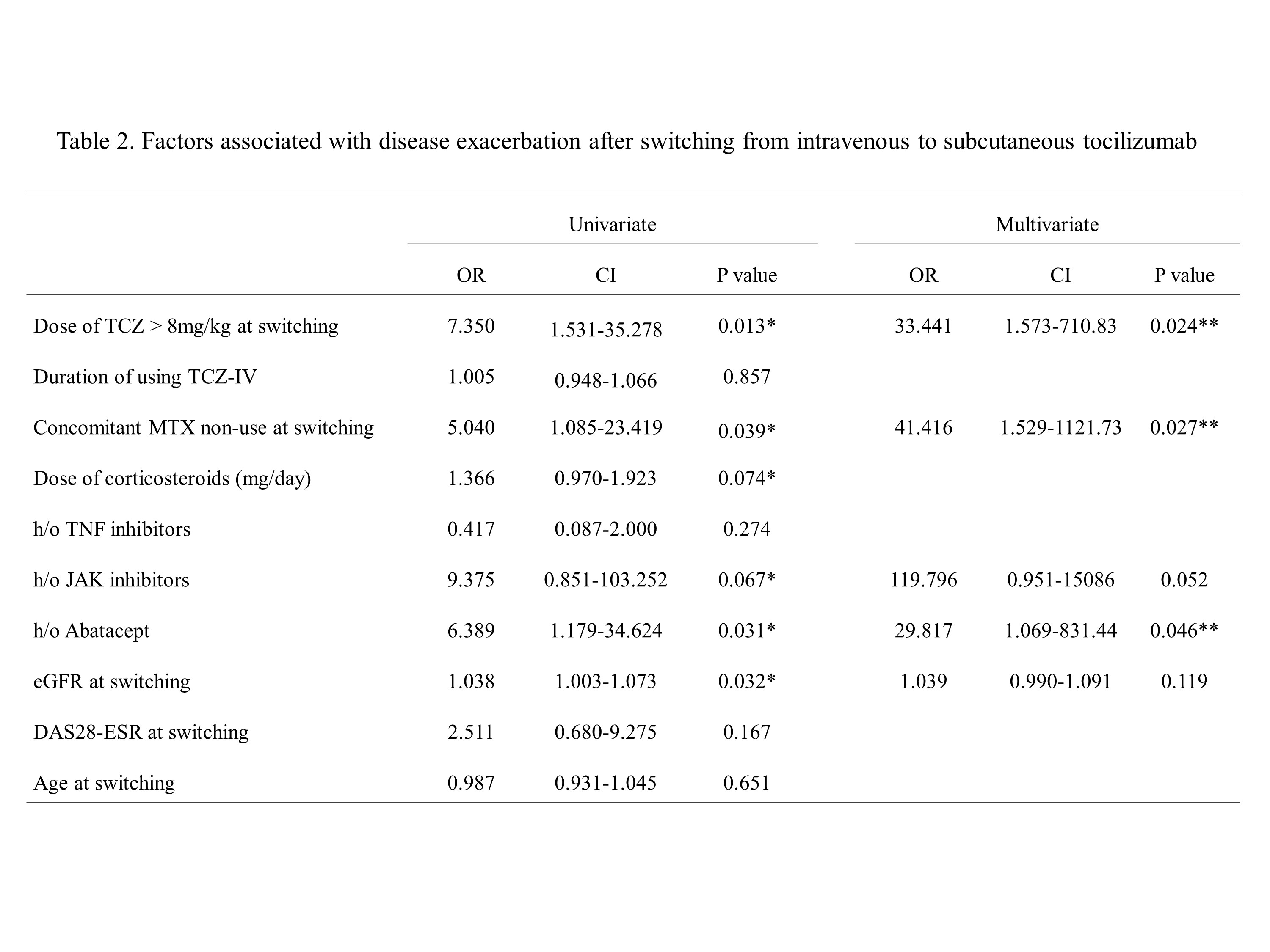
Results: Among 147 patients who were treated initially with TCZ-IV, 37 patients were switched to TCZ-SC after the acquisition of remission or low disease activity. The SC inefficacy and SC efficacy groups included 11 (29.7%) and 26 (70.3%) patients, respectively. At the time of switching, mean DAS28 was not different between the two groups. However, doses of TCZ-IV per weight and methotrexate were higher in the SC inefficacy group. In the multivariate analysis, the use of a high dose (more than 8 mg/kg) TCZ (odds ratio [OR] 33.441, 95% confidence interval [CI], 1.573–710.833, p=0.024), methotrexate non-user(OR 41.416, 95% CI, 1.529–1121.731, p=0.027), and history of prior abatacept use (OR 29.817, 95% CI, 1.069–831.442, p=0.046) were associated with the risk of RA flares after switching to TCZ-SC.
Conclusion: Methotrexate non-use and TCZ-IV overdose per weight were associated with a higher risk of RA flare after switching to TCZ-SC. Thus, we recommend checking these factors before switching from TCZ-IV to TCZ-SC to prevent RA flares.
To cite this abstract in AMA style:
Ahn S, Oh J, Heo H, Hong S, Lee C, Yoo B, Kim Y. Flare After Switching from Intravenous Tocilizumab to Subcutaneous Formulation in Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/flare-after-switching-from-intravenous-tocilizumab-to-subcutaneous-formulation-in-patients-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/flare-after-switching-from-intravenous-tocilizumab-to-subcutaneous-formulation-in-patients-with-rheumatoid-arthritis/