Session Information
Date: Sunday, October 21, 2018
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Long-term apremilast (APR) efficacy and safety were evaluated for up to 5 yrs in adults with active PsA in the phase III PALACE 1-3 studies.
Methods: Subjects were randomized (1:1:1) at baseline (BL) to placebo (PBO), APR 30 mg BID (APR30), or APR 20 mg BID (APR20). PBO subjects were re-randomized (1:1) to APR30 or APR20 at Wk 16 (early escape) or Wk 24. Double-blind APR treatment continued to Wk 52; subjects could continue APR during an open-label, long-term treatment phase for up to an additional 4 yrs. Efficacy and safety were assessed and reported as observed.
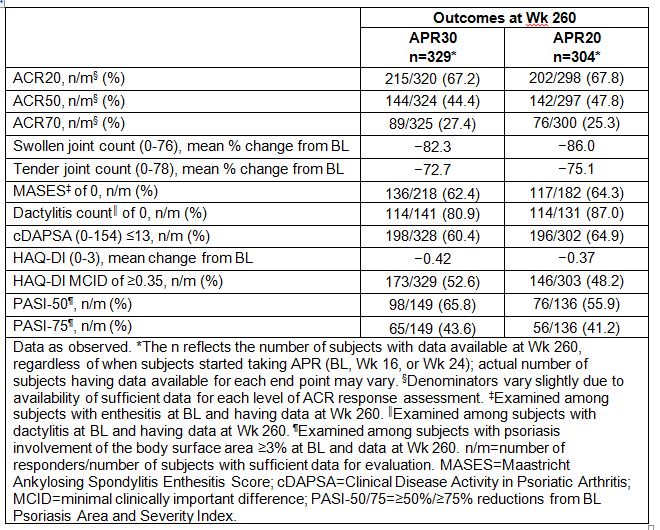
Results: A total of 1,493 subjects were randomized and received ≥1 dose of study medication (PBO: n=496; APR30: n=497; APR20: n=500). Of those randomized to APR30 at BL, 44.5% (221/497) completed 260 wks of treatment. Among APR30 subjects entering Wk 52, 63.2% (331/524) completed 260 wks, regardless of when APR was started (BL, Wk 16, or Wk 24). At Wk 52, ACR20/50/70 response rates were 55.3%/26.1%/11.9% for APR30 subjects. Sustained response rates were observed with continued APR30 treatment at Wk 260 (Table). Marked swollen joint count improvements were seen, with a mean percent reduction of 82.3% at Wk 260; tender joint count reduction was 72.7%. At Wk 260, 62.4% (136/218) of APR30 subjects with BL enthesitis achieved a Maastricht Ankylosing Spondylitis Enthesitis Score of 0; 80.9% (114/141) with BL dactylitis achieved a dactylitis count of 0. A HAQ-DI minimal clinically important difference of ≥0.35 was achieved by 52.6% of APR30 subjects at Wk 260; 60.4% achieved low disease activity or remission, defined as a Clinical Disease Activity in Psoriatic Arthritis score ≤13. Sustained improvements in psoriatic skin involvement were observed with continued treatment at Wk 260 in APR30 subjects with ≥3% BL body surface area involvement, with 65.8% (98/149) and 43.6% (65/149) of subjects, respectively, achieving ≥50%/≥75% reductions from BL Psoriasis Area and Severity Index score. APR 20 results were similar (Table). Consistent efficacy results were seen across the individual studies. During the 0- to ≤52-wk APR-exposure period, adverse events (AEs) occurring in ≥5% of APR30-exposed subjects were diarrhea, nausea, headache, upper respiratory tract infection, and nasopharyngitis. Most diarrhea and nausea AEs were reported within the first 2 wks of treatment and usually resolved within 4 wks; gastrointestinal AE frequency decreased with longer APR30 exposure, and frequency of other common AEs decreased/remained stable with prolonged exposure. Serious AE rate during Wks >208 to ≤260 was 5.8%, consistent with earlier periods.
Conclusion: APR demonstrated sustained, clinically meaningful improvements in PsA signs/symptoms, physical function, and associated psoriasis in the subjects continuing treatment over 5 yrs. APR continued to demonstrate a favorable safety profile and was generally well tolerated at 5 yrs.
To cite this abstract in AMA style:
Kavanaugh A, Gladman DD, Edwards CJ, Schett G, Guerette B, Delev N, Teng L, Paris M, Mease PJ. Five-Year Efficacy and Safety of Apremilast Treatment in Subjects with Psa: A Pooled Analysis of the 3 Phase III Studies [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/five-year-efficacy-and-safety-of-apremilast-treatment-in-subjects-with-psa-a-pooled-analysis-of-the-3-phase-iii-studies/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/five-year-efficacy-and-safety-of-apremilast-treatment-in-subjects-with-psa-a-pooled-analysis-of-the-3-phase-iii-studies/