Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: The symptomatic benefit and NSAID-sparing effects of fish oil (FO) in RA are well known but effects on disease outcomes are less well established, especially in the context of contemporary treatment of early RA. The aim of this investigator-initiated randomized controlled trial was to assess the effects of high vs. low dose FO on disease outcomes in patients with early RA receiving a “treat-to-target” protocol of combination DMARDs.
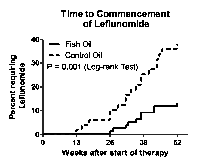
Methods: Patients with RA according to ACR criteria with active polyarthritis of <12 months' duration and who were DMARD-na•ve, received MTX, sulphasalazine and hydroxychloroquine. They were randomized 2:1 to fish oil at a high dose (FO) or low dose (control) providing 5.5 and 0.4 g/day respectively, of the marine omega-3 fats, EPA+DHA. DMARD doses were adjusted according to a pre-defined protocol taking disease activity and toxicity into account. DAS28-ESR, mHAQ, remission and plasma phospholipids were assessed 3 monthly. In a novel study design, the primary outcome was DMARD use at 52 weeks, defined as the addition of leflunomide to triple therapy (“failure of triple therapy”).
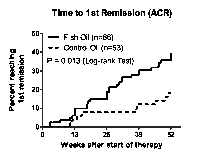
Results: After 52 weeks, there were no significant differences between treatment groups for the outcomes of MTX dose, DAS28 or mHAQ; but compared with controls (16/46, 35%), fewer FO patients (9/75, 12%) had commenced leflunomide (p= 0.005, Fisher’s exact test). In FO patients, the rate of commencement of leflunomide was lower (Hazard Ratio 0.27, 95%CI 0.12-0.59) whereas the rate of achieving first ACR remission was higher (HR 2.22, 95%CI 1.18-4.18) compared with controls (Kaplan-Meier estimate).
There was considerable overlap in plasma omega-3 levels between the FO and control groups. Results were analysed by plasma omega-3 quartiles (t12AUC/unit time). Compared with the lowest quartile, patients in the highest quartile had significantly higher odds of achieving remission, by either DAS28 or ACR criteria (respectively, OR =3.30, 95% CI; 1.13-9.71, OR = 3.53, 95%CI; 1.05-11.90).
Conclusion: FO was associated with benefits additional to those achieved by combination “treat-to-target” DMARDs with similar MTX use. The benefits included reduced likelihood of progression to leflunomide (“failure of triple therapy”) and a higher rate of ACR remission. High plasma n-3 fatty acids were associated with higher odds of achieving remission.
Disclosure:
S. Proudman,
None;
L. Spargo,
None;
C. Hall,
None;
L. McWilliams,
None;
A. Lee,
None;
M. Rischmueller,
None;
R. Gibson,
None;
M. James,
None;
L. G. Cleland,
Melrose Laboratories,
9.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/fish-oil-in-rheumatoid-arthritis-a-randomised-double-blind-trial-comparing-high-dose-with-low-dose/