Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: A previous case control study suggested that hydroxychloroquine (HCQ) might prevent the development of cardiac Neonatal Lupus (cardiac NL) in anti-SSA/Ro antibody (ab) exposed fetuses of SLE mothers. In a subsequent study the use of HCQ reduced the nearly 10 fold increased recurrence rate of cardiac NL in an international cohort of anti-SSA/Ro ab women. Based on these encouraging data, an open label prospective study was initiated to evaluate whether HCQ reduces the recurrence rate of cardiac NL.
Methods: A Phase II trial using a Simon’s two-stage optimal approach was employed to allow for early stopping due to absence of treatment efficacy. In this first stage of the study, 19 anti-SSA/Ro ab pregnant patients with a previous child with cardiac NL (1st degree block excluded) were enrolled and if 3 or more children with cardiac NL were born, the study would be terminated for inefficacy. The protocol called for initiation of HCQ by 10 weeks gestation. Serial echocardiograms (echos) to evaluate for the development of the primary (2nd or 3rd degree block) and secondary (1st degree block) outcomes were performed. Maternal and cord blood HCQ levels were evaluated to assess treatment adherence, pathobiology and efficacy.
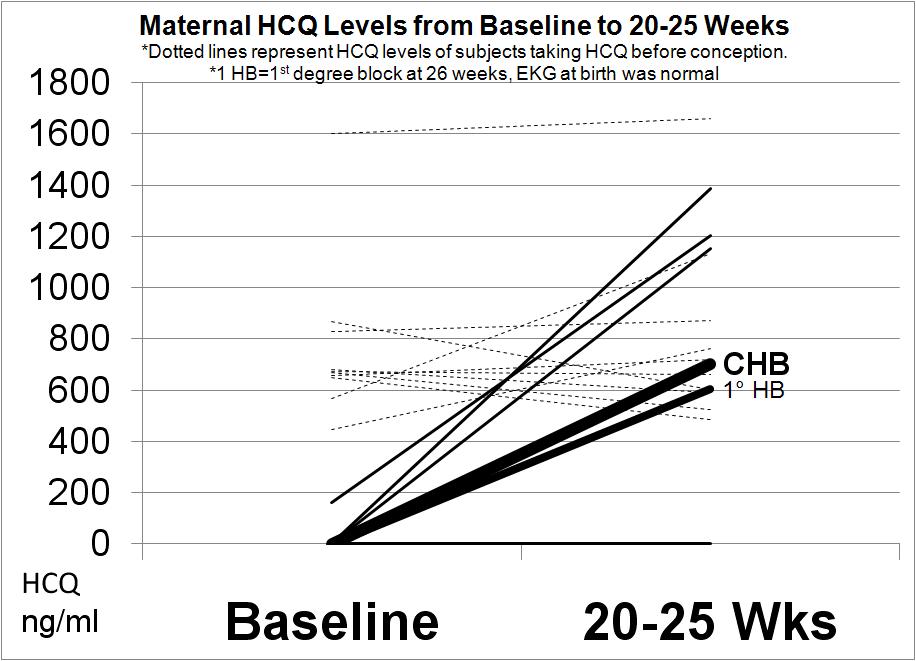
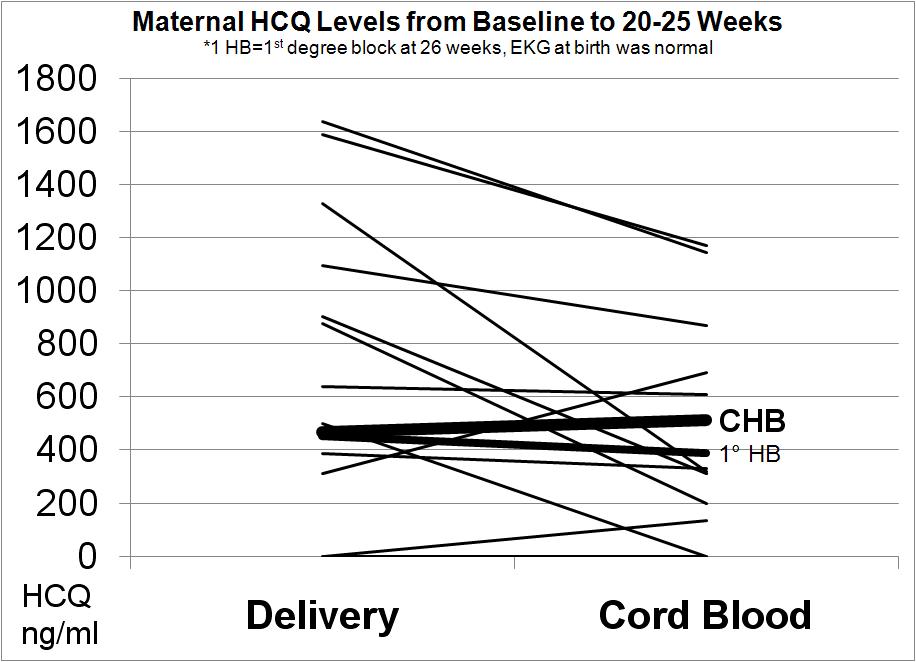
Results: Eighteen pregnancies in 17 women have been completed. Seventeen babies (including a set of twins) had normal serial fetal echos and normal EKGs at birth. One pregnancy resulted in a primary outcome of 3rd degree block. In this pregnancy, the echo at 20 weeks revealed 2nd degree block which progressed 2 days later to 3rd degree. The neonate was not paced until 4.5 months of age. Another pregnancy resulted in a secondary outcome of a prolonged fetal PR interval > 150 msec at 26 weeks for which the mother was treated with 3 days of 4mg dexamethasone. The PR interval normalized and the EKG at birth was normal. In the one ongoing pregnancy, all echos through 37 weeks were normal (notable since the most vulnerable period for complete block is 18-24 weeks). Overall, the rate of recurrence of cardiac NL was 5.3%. Figure 1 reveals that therapeutic HCQ levels can be achieved by the mid second trimester in mothers who were not previously on HCQ prior to conception. Figure 2 substantiates cord blood levels of HCQ.
Conclusion: These prospective data affirm that HCQ may prevent the recurrence of cardiac NL. The feasibility of the strategy using the Simon’s two-stage design was substantiated. Moreover, the study unequivocally demonstrates that measurable HCQ levels can be reached prior to the vulnerable period and are present in the cord blood, reflective of placental transfer.
Disclosure:
P. M. Izmirly,
None;
N. Costedoat-Chalumeau,
None;
A. Saxena,
None;
A. Zink,
None;
Z. Smith,
None;
D. Friedman,
None;
J. P. Buyon,
NIH,
2.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/first-stage-of-a-simons-two-stage-optimal-approach-supports-placental-transfer-of-hydroxychloroquine-and-a-reduced-recurrence-rate-of-the-cardiac-manifestations-of-neonatal-lupus/