Session Information
Date: Tuesday, November 7, 2017
Title: Health Services Research I: Cost Drivers in Rheumatic Disease
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Although bDMARDs are often able to control the RA, they are expensive and associated with adverse effects. Therefore, it is important to use the lowest effective dose in each patient. Several studies have shown that, disease activity-guided dose reduction without deterioration of disease activity is possible, while saving costs in patients with stable and low disease activity.1 Despite these positive results, questions remain on the optimal tapering strategy. Different strategies are conceivable, with varying results regarding the balance between the number of flares, utilities and costs. Therefore, the objective of this study was to investigate the most cost-effective TNFi dose reduction strategy for RA patients using a modelling design.
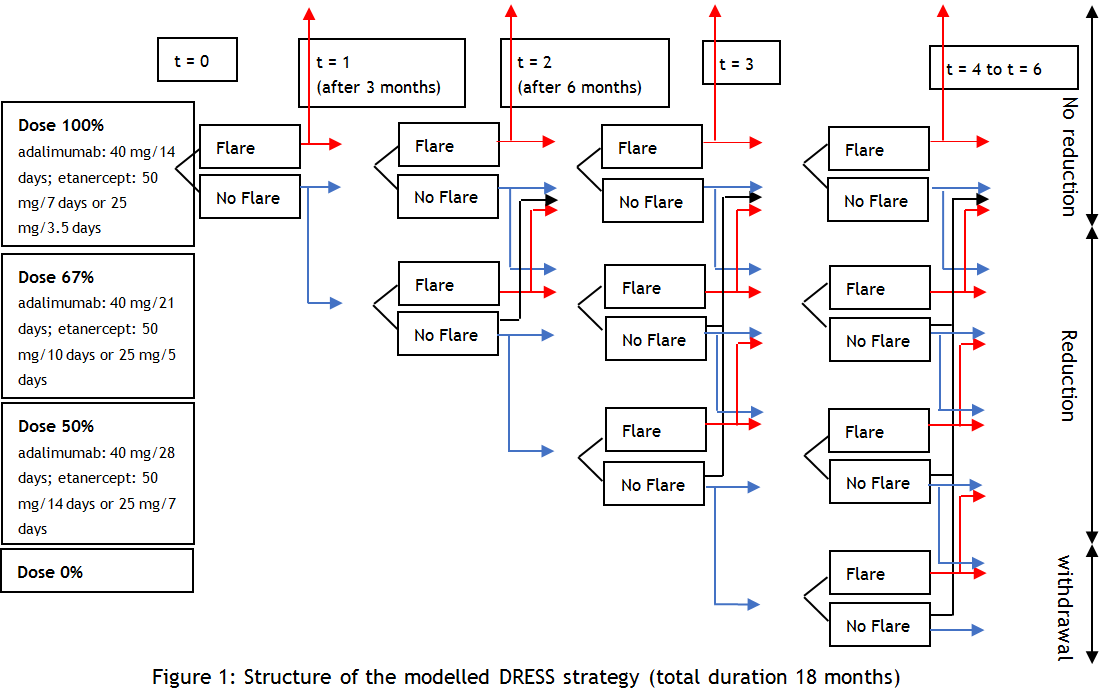
Methods: In a cost-utility analysis using Markov modelling based on data from the DRESS study, STRASS study, and the RA Nijmegen cohort, the following strategies were tested: 1. four steps DRESS tapering (figure 1: 100%-67%-50%-0%); 2. Tapering with an extra dosage step of 33%; 3. Tapering without withdrawal; 4. Use of a stricter flare criterion; and 5. Use of a predictor (biomarker: 80% specific, 80% sensitive, €100 per test) for successful tapering. Also, a continuation group (strategy 0) was modelled and used as comparator. Scenario analyses with 30% and 50% drug price discount (biosimilars) and no discounting were executed. In addition, it was examined how well a biomarker should be able to predict to become cost-effective.
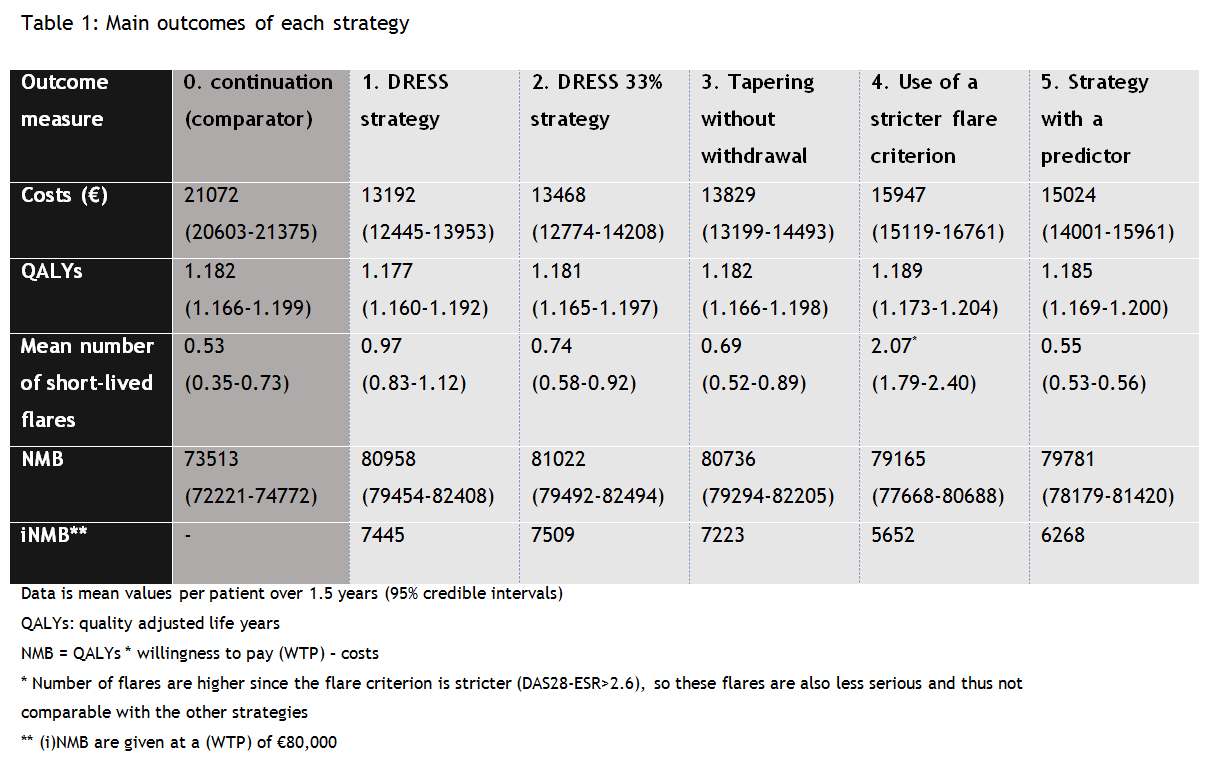
Results: All examined tapering strategies were found to be cost saving, but yielded more short-lived flares compared to strategy 0. The change in utilities was minimal (large overlap in credible intervals) (table 1). Strategy 2 is cost-effective compared to all other strategies (highest incremental net monetary benefit (iNMB)). Due to the large overlap in credible intervals of the NMBs of strategy 1, 2 and 3, the chance that they are actually not different in terms of cost-effectiveness is high. Scenario analyses did not change results. A biomarker becomes cost-effective when it has a sensitivity and specificity of 96% or higher.
Conclusion: All dose reduction strategies dominated the continuation strategy. For use in clinical practice, we recommend a choice between strategy 1, 2 and 3, based on shared decision making.
1. Nam et al, Ann Rheum Dis. 2017 Jun;76(6):1113-1136
To cite this abstract in AMA style:
Bos DPG, Verhoef LM, van den Ende CHM, van den Hoogen FHJ, Fautrel B, Hulscher MEJL, Kievit W, Den Broeder AA. Finding the Optimal Treatment Strategy for Disease Activity-Guided Dose Reduction of Adalimumab and Etanercept in Rheumatoid Arthritis: A Modelling Study [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/finding-the-optimal-treatment-strategy-for-disease-activity-guided-dose-reduction-of-adalimumab-and-etanercept-in-rheumatoid-arthritis-a-modelling-study/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/finding-the-optimal-treatment-strategy-for-disease-activity-guided-dose-reduction-of-adalimumab-and-etanercept-in-rheumatoid-arthritis-a-modelling-study/