Session Information
Date: Sunday, November 13, 2022
Title: RA – Treatment Poster II
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: BAT1806 (also referred to as BIIB800) is a proposed biosimilar to tocilizumab reference product (TCZ). Results of this Phase 3, randomized, double-blind, active-controlled trial demonstrated that BAT1806 has equivalent efficacy and comparable safety, immunogenicity, and pharmacokinetic (PK) profiles as TCZ up to Week 24 (treatment period 1).1 Here, the results from Weeks 24 to 48 (treatment period 2 [TP2]) are reported.
Methods: The study was conducted in 55 sites in China and Central Europe. Subjects with a diagnosis of RA according to the ACR/EULAR 2010 revised classification for at least 6 months prior to screening and who met the protocol-specified inclusion/exclusion criteria were randomized in a 2:1:1 ratio to one of three treatment groups: (1) BAT1806 up to Week 48, (2) TCZ up to Week 48, or (3) TCZ up to Week 24 followed by BAT1806 from Week 24 to Week 48, administered intravenously every 4 weeks at a dose of 8 mg/kg. Efficacy in terms of ACR20/50/70 response probabilities and DAS28-ESR and DAS28-CRP, PK, safety, and immunogenicity were assessed up to Week 48.
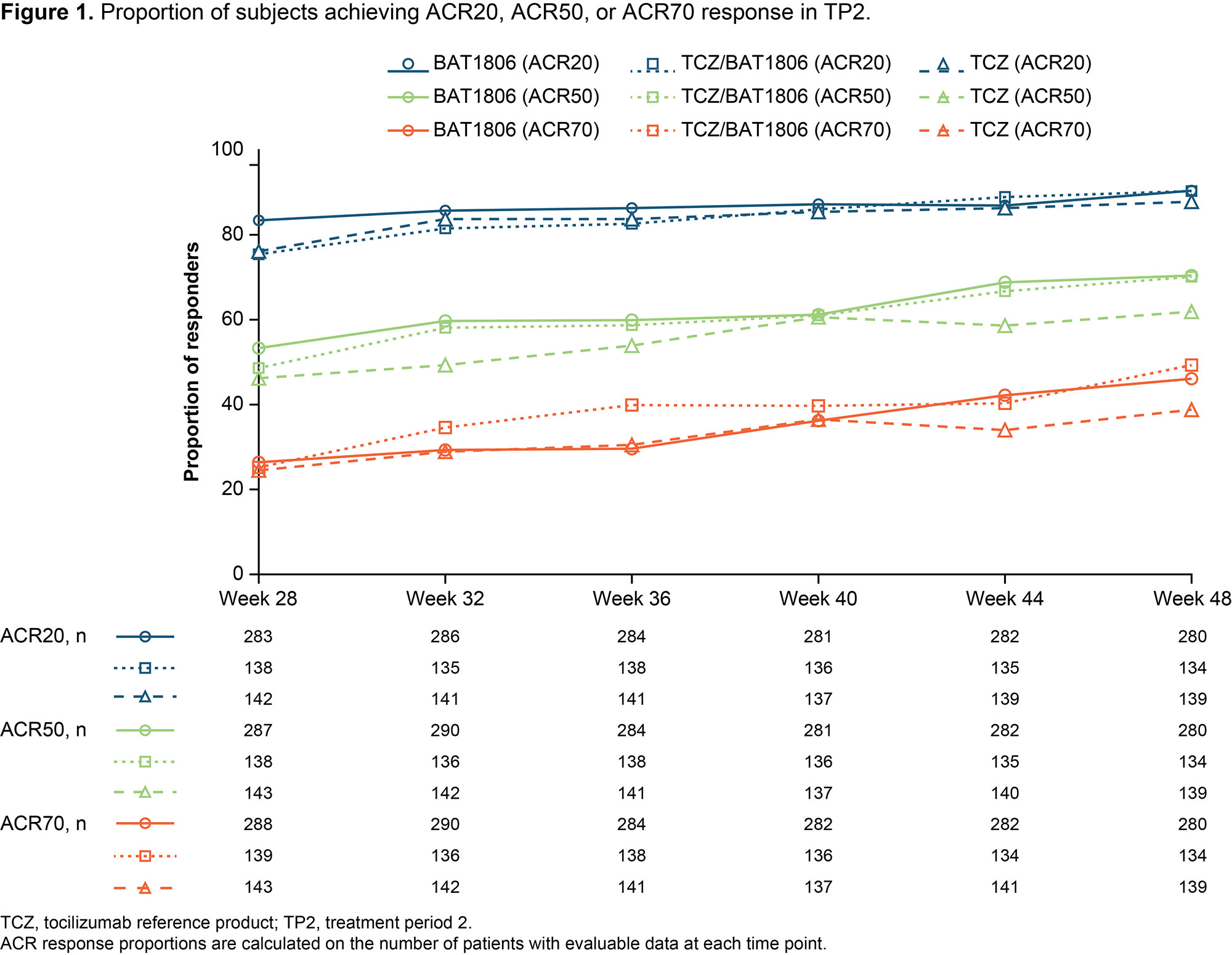
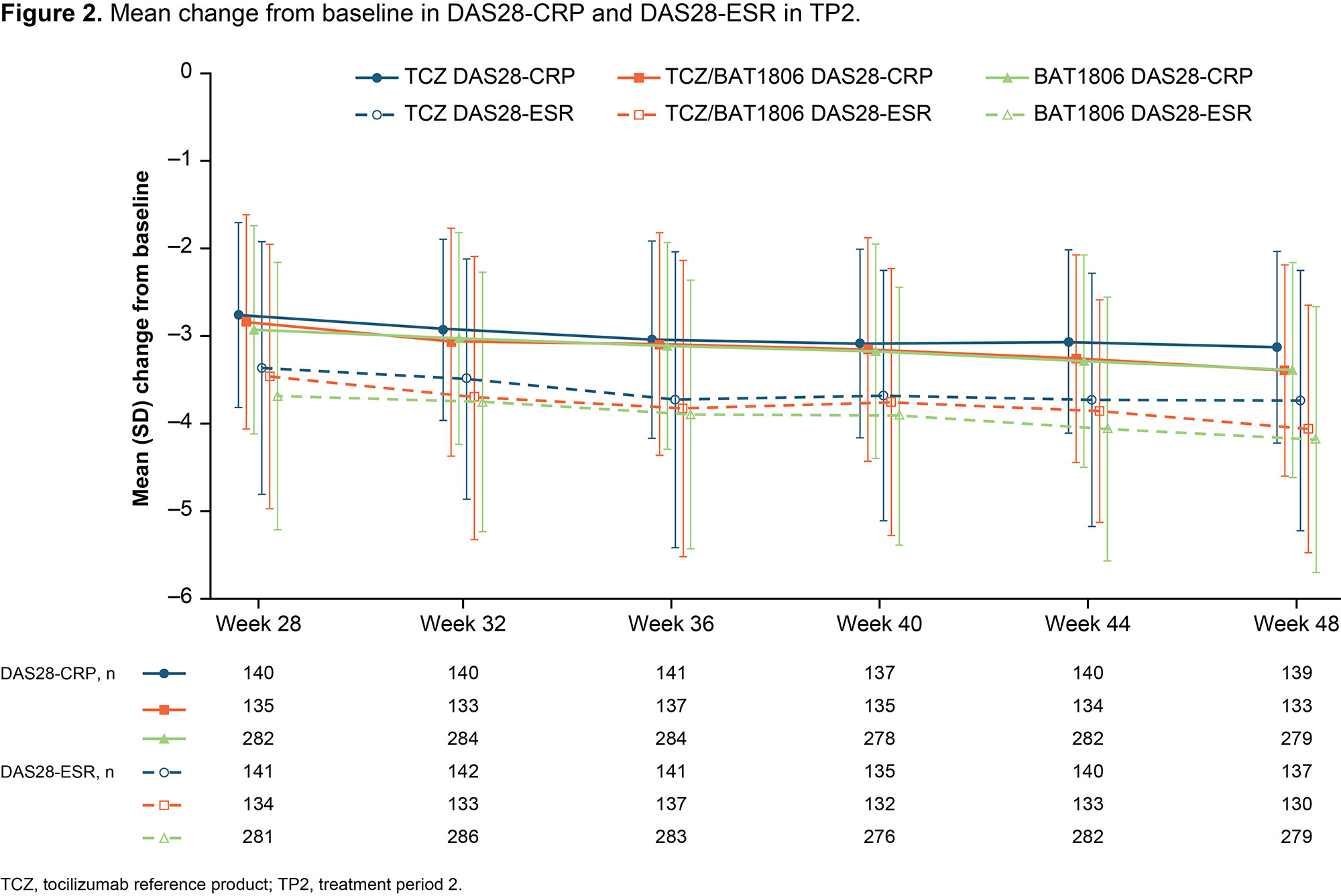
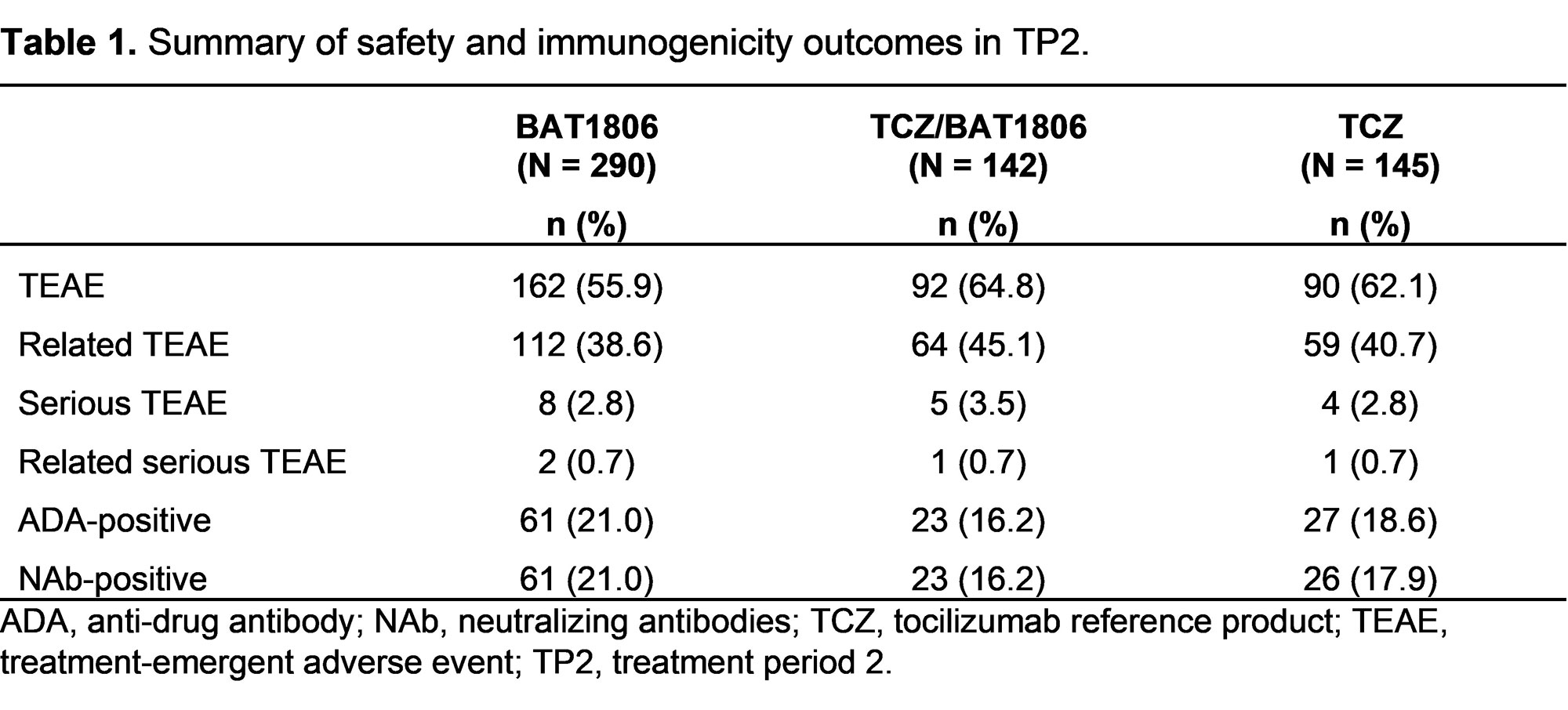
Results: Of 621 randomized subjects, the numbers of subjects continuing into TP2 were 290 (92.9%), 142 (92.2%), and 145 (93.5%) in groups 1, 2, and 3, respectively. Baseline characteristics were similar across groups at Week 24. At Week 48, ACR20 responders as proportions of evaluable subjects were: 253/280 (90.4%), 121/134 (90.3%), and 122/139 (87.8%) for groups 1, 2, and 3, respectively. ACR20/50/70 responses (Figure 1) and mean change in DAS28-ESR and DAS28-CRP (Figure 2) from baseline were comparable across groups. The proportions of subjects with serious treatment-emergent adverse events (TEAEs) in TP2 were similar between the treatment groups (Table 1). No deaths were reported during TP2. Eight subjects in group 1 reported 9 serious TEAEs, 2 of which (pneumonia and salpingo-oophoritis) were possibly treatment related. Five subjects in group 2 reported 5 serious TEAEs, 1 of which was possibly treatment related (laryngitis). Four subjects in group 3 reported 5 serious TEAEs, 1 of which was possibly treatment related (pneumonia). Anti-drug antibodies were reported at least once in 21.0%, 16.2%, and 18.6% of subjects in treatment groups 1, 2, and 3, respectively; of these subjects, all but 1 in the TCZ group tested positive for neutralizing antibodies (Table 1). Geometric mean (CV%) trough serum concentrations (µg/mL) were comparable for the 3 treatment groups: 13.1 (126.5), 12.7 (157.0), 12.4 (180.7) at Week 28 and 12.3 (82.7), 13.4 (89.2), 13.5 (130.6) at Week 44 for groups 1, 2, and 3, respectively. The study was conducted between 2019–2020 during the COVID-19 pandemic. Post-hoc exploratory analyses indicated that the pandemic had limited impact, which was similar between the treatment groups (data not shown).
Conclusion: Efficacy, safety, immunogenicity, and PK profiles were comparable between BAT1806, TCZ/BAT1806, and TCZ throughout TP2. No safety or clinically relevant immunogenicity issues were observed in subjects switched from TCZ to BAT1806
Reference
1. Leng X, et al. Ann Rheum Dis. 2022;81(suppl. 1):388.
To cite this abstract in AMA style:
Leng X, Leszczynski P, Jeka S, Liu S, Liu H, Miakisz M, Gu J, Kilasonia L, Stanislavchuk M, Yang X, Zhou Y, Dong Q, Mitroiu M, Addison J, Zeng X. Fifty-two-week Results from a Phase 3, Randomized, Double-blind, Active-controlled Clinical Trial to Compare BAT1806/BIIB800, a Proposed Tocilizumab Biosimilar, with a Tocilizumab Reference Product in Subjects with Moderate to Severe RA with an Inadequate Response to Methotrexate [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/fifty-two-week-results-from-a-phase-3-randomized-double-blind-active-controlled-clinical-trial-to-compare-bat1806-biib800-a-proposed-tocilizumab-biosimilar-with-a-tocilizumab-reference-product-in/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/fifty-two-week-results-from-a-phase-3-randomized-double-blind-active-controlled-clinical-trial-to-compare-bat1806-biib800-a-proposed-tocilizumab-biosimilar-with-a-tocilizumab-reference-product-in/