Session Information
Date: Wednesday, October 29, 2025
Title: Abstracts: Systemic Sclerosis & Related Disorders – Clinical III (2651–2656)
Session Type: Abstract Session
Session Time: 10:30AM-10:45AM
Background/Purpose: Pregnancy in women with systemic sclerosis (SSc) is considered high-risk due to complications like scleroderma renal crisis and preeclampsia, related to vasculopathy. Prospective data on pregnancy outcomes in SSc are lacking. We aimed to describe adverse pregnancy outcomes (APOs), disease course, and associated factors in SSc pregnancies, and to compare APO rates to the general population.
Methods: We included all pregnancies in women with SSc (ACR/EULAR 2013 criteria) or very early SSc (VEDOSS) enrolled in the French prospective GR2 study (2014–2020). We analyzed pregnancies beyond 22 weeks for APOs: fetal demise, preterm delivery≤34 weeks, placental insufficiency complications (preeclampsia, HELLP, fetal growth restriction [FGR], placental abruption), and small for gestational age (SGA, < 10th percentile using AUDIPOG curves). Disease worsening was defined as the appearance or progression of vascular manifestations (cutaneous ulcers, pulmonary arterial hypertension, or renal crisis), interstitial lung disease, worsening of skin fibrosis, calcinosis, joint involvement, or gastrointestinal tract involvement (excluding gastroesophageal reflux disease). Adverse pregnancy outcome rates were compared to those of age-matched controls from the 2016 French perinatal survey.
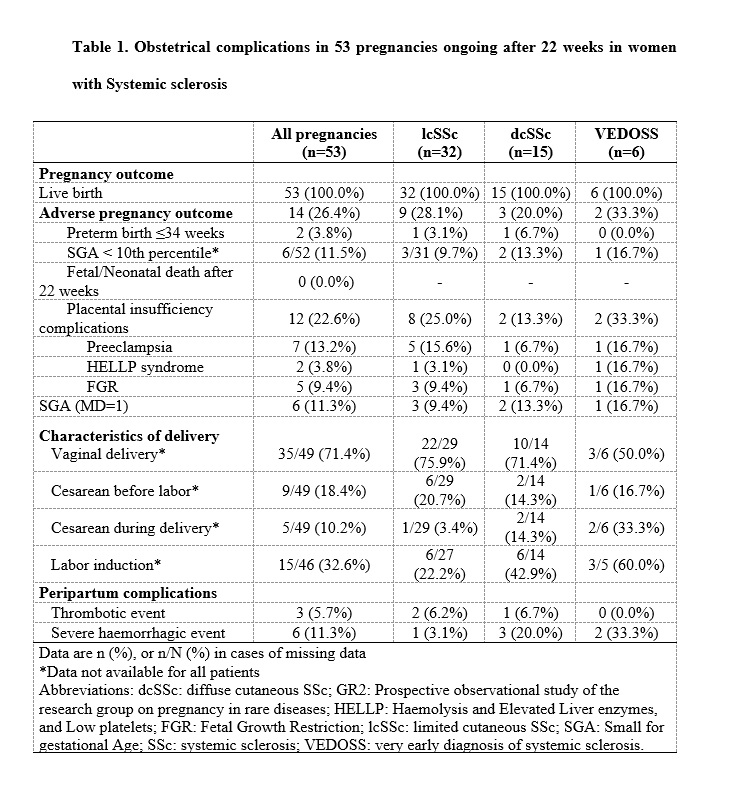
Results: Fifty-eight pregnancies (52 women) were included: 16 diffuse SSc (27.5%), 34 limited (58.6%), and 8 VEDOSS (13.8%). None had prior pulmonary arterial hypertension or renal crisis. Among 53 pregnancies beyond 22 weeks, 14 (26.4%) had APOs: 2 preterm deliveries (3.8%), 12 placental complications (22.6%; 7 preeclampsia, 5 FGR), and 6 SGA (11.3%). Six women (11.3%) had severe postpartum hemorrhage. Rates of preeclampsia, preterm birth, birth weight < 2500g, and postpartum hemorrhage were significantly higher than in the general population (13.2% vs 3.0%, p=0.001; 13.2% vs 5.8%, p< 0.047; 21.1% vs 4.3%, p< 0.0001; 11.3% vs 1.4%, p< 0.0001). No factors were significantly associated with APO in univariate analysis.Disease worsened in 23 of 58 pregnancies (39.7%), mostly cutaneous vascular events, especially postpartum. Risk factors included absence of anti-centromere antibodies (OR=0.2 [0.1–0.8]), diffuse SSc (OR=3.7 [1.1–12.4]), and prior cutaneous vascular involvement (OR=3.7 [1.2–11.5]).
Conclusion: In this prospective cohort of 58 pregnancies in women with SSc or VEDOSS and no prior severe organ involvement, 91.4% resulted in live births. Despite a relatively low incidence of severe complications, SSc pregnancies were associated with significantly higher rates of APOs and severe postpartum hemorrhage compared to the general population. Disease progression occurred in nearly 40% of cases, particularly affecting skin vascular manifestations during the postpartum period, and was more frequent in women with diffuse cutaneous SSc, prior vascular involvement, and antibodies other than anti-centromere.
To cite this abstract in AMA style:
Murarasu A, beaudeau l, Le Guern V, guettrot-Imbert g, cazalets c, durant c, Yelnik C, Roussin C, Besse M, Berthoux E, Chatelus E, Hachulla E, LAZARO E, Maurier F, leroux G, Pugnet G, durieu I, RAFFRAY L, Le Besnerais M, roriz M, SOUCHAUD-DEBOUVERIE O, JEGO P, ORQUEVAUX P, de Moreuil C, MAILLARD H, Morel N, Molto A, Le Ray C, pannier e, sentilhes l, Mouthon L, Costedoat-Chalumeau N, Chaigne B. Fetal and maternal outcomes in systemic sclerosis and very early diagnosis of systemic sclerosis pregnancies, a national prospective study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/fetal-and-maternal-outcomes-in-systemic-sclerosis-and-very-early-diagnosis-of-systemic-sclerosis-pregnancies-a-national-prospective-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/fetal-and-maternal-outcomes-in-systemic-sclerosis-and-very-early-diagnosis-of-systemic-sclerosis-pregnancies-a-national-prospective-study/

.jpg)
.jpg)