Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: A very serious drug-related adverse event is reported with inhibitors of IL-1 and IL-6 (IL-1i/IL6i). This reaction scores as drug reaction with eosinophilia and systemic symptoms (DReSS) and strongly associates with HLA-DRB1*15 (Saper Ombrello 2022). Since these HLA alleles incompletely predict reaction risk, we compare demographics, timing of macrophage activation syndrome (MAS), reaction associated features, and survival for reaction subjects with and without DRB1*15 alleles.
Methods: Inclusion required HLA genotyped Still/Still-like disease subjects scoring as DReSS per RegiSCAR for DReSS (Kardaun 2013) implicating anakinra, canakinumab, rilonacept, or tocilizumab. Clinical data were from Still onset through spring 2023 or, if deceased, until death. MAS was per Ravelli 2016. Centers avoiding IL-1/IL-6-inhibitors in subjects with HLA-DRB1*15 were allowed to participate. Demographics, onset features, reaction-related complications and timings, and survival were compared between subjects with and without HLA-DRB1*15 alleles. A European control group with DRB1*15 genotype information was included for comparison (Osoegawa 2021). Continuous comparisons were by Mann-Whitney rank test, proportions by Fisher exact, and event free probability based on Kaplan Meier estimate.
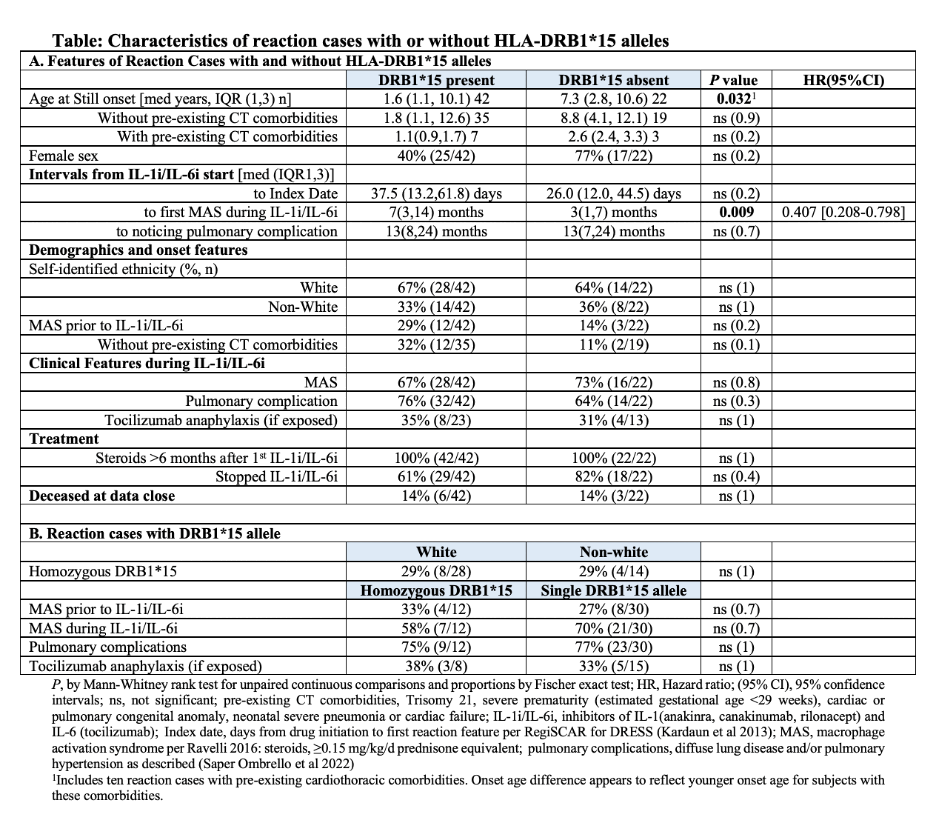
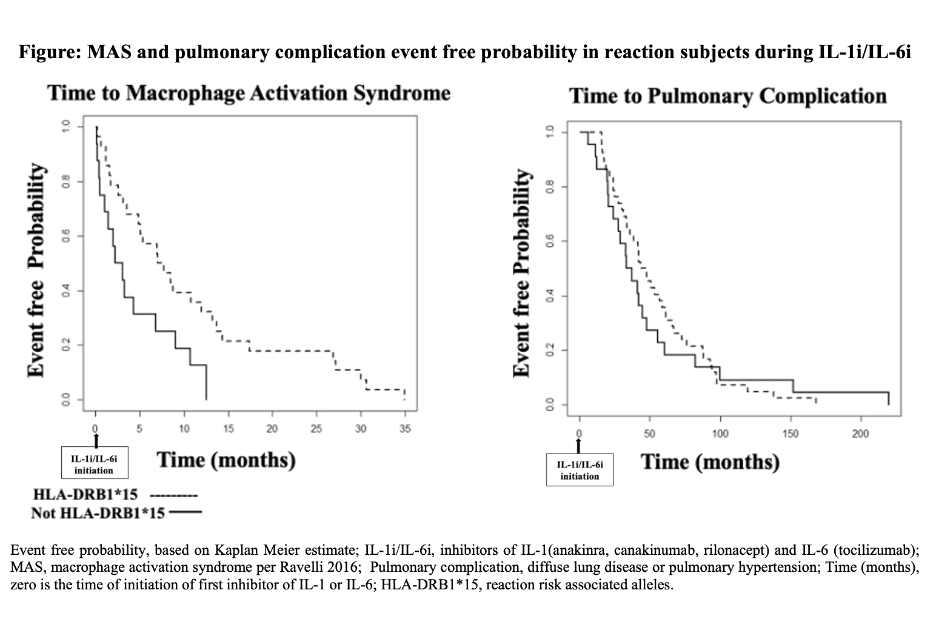
Results: 42 subjects were DRB1*15 and 22 were not-DRB1*15. “Definite” DReSS score was reported in 62 subjects, and “probable” DReSS score was reported in two subjects (one DRB1*15, one not-DRB1*15). Homozygous DRB1*15 was present in 29%(8/28) of white subjects, contrasting with 1.5%(30/2029) of European control subjects, [p=3.5×10-7, OR(95%CI)16.2(6.0,39.6)]. Except for finding a shorter time interval from drug initiation to initial episode of MAS during IL-1i/IL-6i for those without DRB1*15, demographics, onset features, reaction features, and survival were not significantly different between subjects with and without DRB1*15 alleles. (Table and Figure)
Conclusion: HLA-DRB1*15 associated IL-1i/IL-6i-reaction-risk is strengthened by finding enrichment for homozygosity. Should a reaction scoring as DReSS occur, Still’s disease severity, reaction expression and survival appear not to differ based on presence or absence of DRB1*15. Younger age at onset of Still disease appears to associate with pre-existing cardiothoracic comorbidities independent of HLA-DRB1*15. Vigilance for this severe delayed reaction appears indicated when IL-1i/IL-6i are used, shown here in Still and Still-like illness, even in the absence of DRB1*15.
To cite this abstract in AMA style:
Saper V, Osoegawa K, Verstegen R, Fernandez Vina M, Tian L. Features of interleukin-1 and interleukin-6 Inhibitor (IL-1i/IL-6i) Related Severe Delayed Adverse Reactions in Subjects with or Without Reaction-risk Associated HLA-DRB1*15 [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/features-of-interleukin-1-and-interleukin-6-inhibitor-il-1i-il-6i-related-severe-delayed-adverse-reactions-in-subjects-with-or-without-reaction-risk-associated-hla-drb115/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/features-of-interleukin-1-and-interleukin-6-inhibitor-il-1i-il-6i-related-severe-delayed-adverse-reactions-in-subjects-with-or-without-reaction-risk-associated-hla-drb115/