Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Hydroxychloroquine (HCQ) is a generally safe and widely used rheumatologic drug. Maculopathy is an adverse effect in < 1% in the first 5 years of use; risk rises with exposure and presence of comorbidities. The American Academy of Ophthalmology (AAO) screening guidelines for HCQ maculopathy recommend a baseline within the 1st year and, if normal, annual screening after 5 years. Patients are screened at baseline with a fundus examination at minimum and per the provider’s discretion may also undergo additional testing.
We evaluated our institutional screening practices and determined the cost of excess services provided and whether this practice led to an increase in detection of toxic maculopathy. We hypothesize that patients in our system are over screened, leading to waste.
Methods: All patients with an HCQ prescription in their medication list were included. Exclusions were: macular disease, single prior prescription, never-users or those with adverse effect with < 3 months of use, and those without appointments with rheumatology, ophthalmology or primary care within the institution. Electronic chart-based data collection included: weight-based dose of HCQ, duration of treatment (< 1, 1-5, or >5 years), tamoxifen use, history of kidney disease, and presence of toxic maculopathy. Screening recommendations were evaluated in compliance with the AAO recommendations for baseline and follow-up screening.
Results: 463 patients were prescribed HCQ between January 2010 and April 2020; 157 were included. HCQ use duration was distributed into 3 groups: < 1 year (n= 41), 1-5 years (n= 59), and >5 years (n= 57).
Of the 157 included patients, 38.7% underwent baseline ophthalmologic exam within one year of starting HCQ, 27.7% did not, and 33.6% had no documentation of a retinal exam. 33.6% of patients were inappropriately recommended to be screened again after baseline exam within 1 year. Only 2% of patients were advised to rescreen following baseline exam after 5 years exposure per AAO guidelines. 9% of patients on HCQ for more than 5 years were appropriately receiving annual screening; 12.2% were not. All 3 cases of HCQ maculopathy in our system were diagnosed in appropriately screened patients. Over screening resulted in an additional $1853 per patient.
Conclusion: Our analysis of institutional screening practices for HCQ reveal that only 41% of patients are being appropriately screened. Contrary to our hypothesis, of the inappropriately screened patients, 29% were over screened and 71% were under screened. Of patients who had recorded follow-up recommendations after baseline exam, 94% were inappropriately recommended to follow up in 1 year, while only 6% were recommended to follow up after 5 years of HCQ use per AAO guidelines. Insurance demands regarding specialty care may be a cause of over screening as follow up visits greater than one year out require more administrative burden. Limitations to this study include inadequate available data and reliance on clinical documentation to establish screening methods. In those over screened, utilization costs were high, an additional $1853/patient. For those under screened, the health and financial consequences remain unknown.
 Table 1: Demographics of included patients on hydroxychloroquine. CKD = Chronic Kidney Disease
Table 1: Demographics of included patients on hydroxychloroquine. CKD = Chronic Kidney Disease
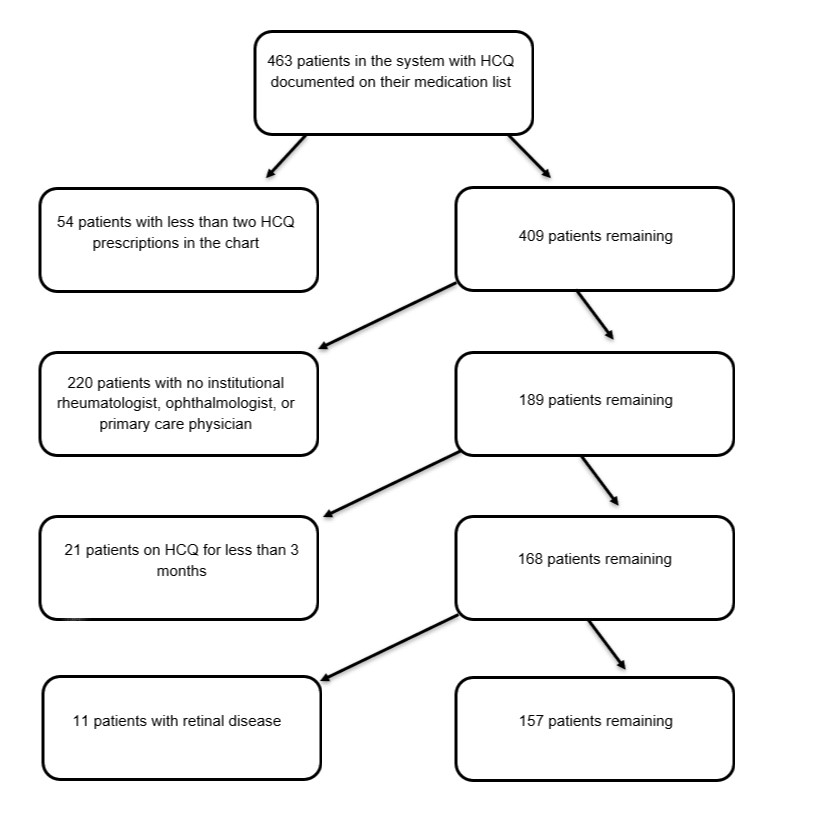 Figure 1: Diagram of method of patient exclusion from study
Figure 1: Diagram of method of patient exclusion from study
 Table 2: Screening practices and follow up recommendations for patients divided into length of time on hydroxychloroquine (HCQ)
Table 2: Screening practices and follow up recommendations for patients divided into length of time on hydroxychloroquine (HCQ)
To cite this abstract in AMA style:
James A, Kam K, Sandhu V, Downey C. Feast or Famine? An Institutional Assessment of Hydroxychloroquine Screening Practices [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/feast-or-famine-an-institutional-assessment-of-hydroxychloroquine-screening-practices/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/feast-or-famine-an-institutional-assessment-of-hydroxychloroquine-screening-practices/
