Session Information
Date: Monday, October 27, 2025
Title: (1467–1516) Systemic Lupus Erythematosus – Diagnosis, Manifestations, & Outcomes Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: The concept of disease modification (DM) has been recently introduced to improve the long-term care of patients (pts) with SLE. DM is defined as ‘the minimization of disease activity with the fewest treatment (tx)-associated toxicities and slowing/preventing organ damage progression.’ To claim DM ultimately requires delayed or prevented progression of organ damage beyond 5 years. The concept of DM is valuable for assessing ‘whether an intervention is on track for achieving DM at the 5-year mark,’ based on interim evaluations during the first 5 years. Latin American pts with SLE are prone to poorer outcomes, which are associated with both disease severity and social determinants of health (SDH). This preliminary study evaluates the prevalence of extrarenal DM in Latin American pts with SLE and examines differences in SDH and tx between those who did and did not achieve extrarenal DM.
Methods: GLADEL 2.0 is a longitudinal cohort assessing the incidence and prevalence of SLE. Forty-three centers from 10 Latin-American countries enrolled pts ≥18 years of age who fulfilled the 1982/1997 ACR and/or the 2012 Systemic Lupus International Collaborating Clinics (SLICC) classification criteria for SLE. The composite definition of DM for extrarenal SLE included: (i) a significant reduction in SLE Disease Activity Index (SLEDAI; >3 points), (ii) no severe flares, and (iii) ≤10 mg/day of prednisone for months 0–12 and ≤5 mg/day prednisone-equivalent for years 2–5. Pts with complete data at baseline, 12, 24, 36, 48, and 60 months were analyzed. We compared baseline SDH and exposure to medications between pts who did and did not achieve DM criteria over time.
Results: Among 1083 pts who entered the GLADEL 2.0 cohort, 709 had the baseline data needed to fulfill DM criteria. Table 1 shows differences in demographic and SDH data by extrarenal DM achievement groups over time. Data from month 60 were not analyzed as data were available for only 64 pts. Although educational attainment varied between groups and genders at each time point, a higher proportion of pts achieved DM among men compared to women at the 36 and 48-month marks. Overall, the proportion of pts achieving DM criteria ranged between 16%–20% across time points (Fig 1a). Figure 1b shows DM trajectories during follow-up. Medication exposure is depicted in Fig 2. A higher number of pts achieving extrarenal DM at months 36 and 48 received cyclophosphamide.
Conclusion: In this preliminary investigation, up to 20% of Latin American pts with SLE achieved extrarenal DM. The study highlights critical disparities, indicating that pts with lower educational attainment and women were less likely to achieve DM. Our findings suggest that specific medication exposure plays a significant role in extrarenal DM achievement, particularly with medications like cyclophosphamide. These results underscore the importance of addressing SDH and tailoring tx strategies to improve outcomes for marginalized groups within this population. Continued research is essential to discern the independent contributions of SDH and different tx modalities on achieving extrarenal DM, ultimately aiming to enhance long-term care and pt quality of life.
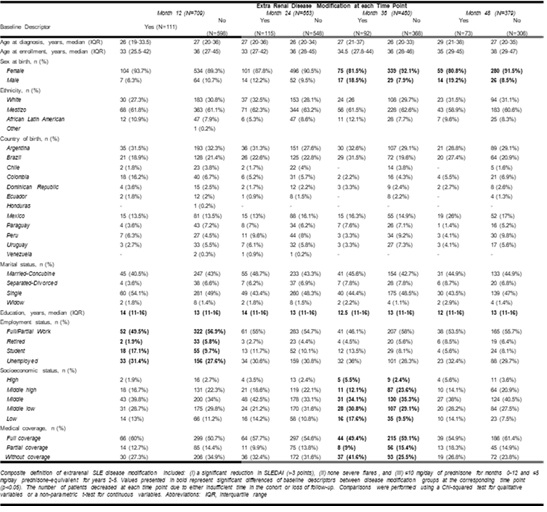 Table 1. Baseline sociodemographics and their differences among patients with SLE who did and did not achieve extrarenal disease modification over time
Table 1. Baseline sociodemographics and their differences among patients with SLE who did and did not achieve extrarenal disease modification over time
.jpg) Figure 1. Percentage of patients achieving potential for extrarenal SLE disease modification (A) and disease modification trajectory (B)
Figure 1. Percentage of patients achieving potential for extrarenal SLE disease modification (A) and disease modification trajectory (B)
.jpg) Figure 2. Medication exposure in patients who did (A) and (B) did not achieve DM
Figure 2. Medication exposure in patients who did (A) and (B) did not achieve DM
To cite this abstract in AMA style:
Barahona-correa j, Bernal-Macías S, Fernandez D, Muñoz Ó, Hernández L, Palacios Santillan E, Maurelli L, Alba P, Saurit V, Garcia L, Sattler M, Bertolaccini M, Micelli M, Gomez G, Cosatti M, Ralle A, Serventi J, Silva A, MONTICIELO O, Duarte Á, Alves Alvino L, Borba E, Bonfa E, dos Reis-Neto E, Guerra Herrera I, Mimica M, Aroca Martínez G, Gómez Escorcia L, Cañas C, Quintana-Lopez G, Toro-Gutierrez C, Martínez Pérez J, Sánchez-Briones R, Pérez Cristóbal M, Martin-Nares E, Juarez-Vicuña Y, García-Valladares I, Hernández R, Esquivel Valerio J, Acosta M, Paats A, Calderón J, Ugarte-Gil M, Calvo A, Alvarez Santana R, Cánepa A, Pizzarossa C, Zazzetti F, Orillion A, Drenkard C. Feasibility of Extrarenal Systemic Lupus Erythematosus Disease Modification in GLADEL 2.0, a Latin American Cohort [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/feasibility-of-extrarenal-systemic-lupus-erythematosus-disease-modification-in-gladel-2-0-a-latin-american-cohort/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/feasibility-of-extrarenal-systemic-lupus-erythematosus-disease-modification-in-gladel-2-0-a-latin-american-cohort/
