Session Information
Session Type: Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: To assess outcomes related to Lupus Therapeutics’ Patient Advocates for Lupus Studies (LT-PALS) a peer-to-peer clinical trial (CT) education program designed to improve CT awareness, knowledge, and enrollment, with a focus on ensuring diverse representation in lupus CTs (LCTs). Lupus patients who had experience participating in a CT were trained as peer educators (PALs) to provide trial agnostic education about CTs to CT-naïve lupus patients. Goals of LT-PALS are to increase awareness and potential risk/benefit of LCTs among a diverse population of patients with lupus; to empower them to make informed decisions about CT participation; and to increase diversity in LCTs to better reflect affected populations.
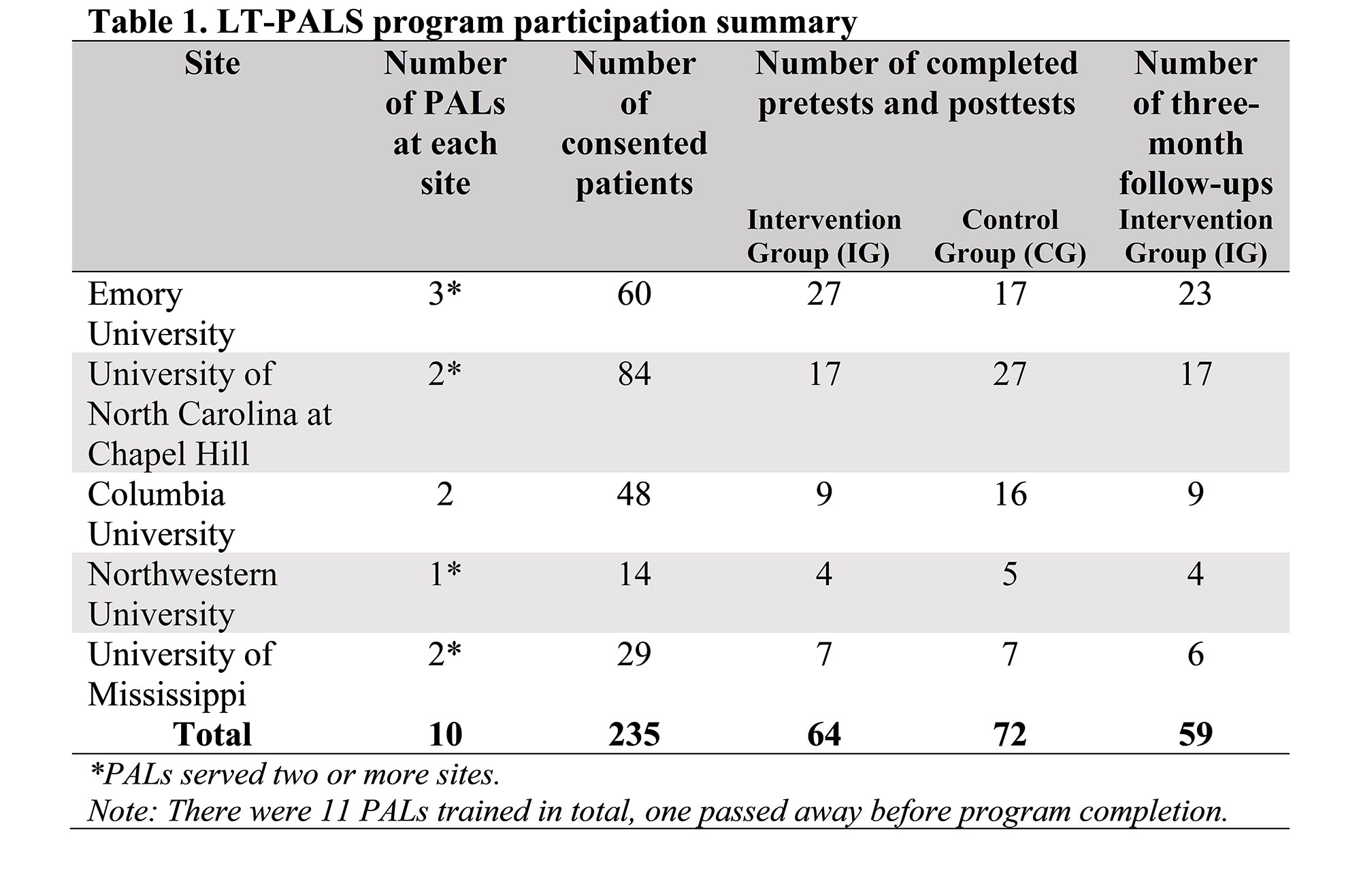
Methods: We used a two-arm, randomized pretest/posttest/follow-up study design to evaluate four cognitive outcomes related to participation in CTs: Knowledge, Attitudes, Self-Efficacy, and Intentions. Five academic medical centers from the Lupus Clinical Investigator’s Network that serve diverse populations piloted the program. PALs completed online and in-person trainings about lupus, CTs, health disparities, and mentoring methodology. The intervention group (IG) received education sessions with trained PALs while the control group (CG) experienced a 3-week waiting period between pretest and posttest. We conducted between group t-tests and multiple linear regressions with posttest scores as dependent variables and participation in the LT-PALS program as the main exposure variable. The IG had a 3-month follow-up which we compared to pretest and posttest scores to assess the sustainability of gains associated with LT-PALS participation. We also gathered qualitative data regarding participants’ concerns/barriers related to CT participation.
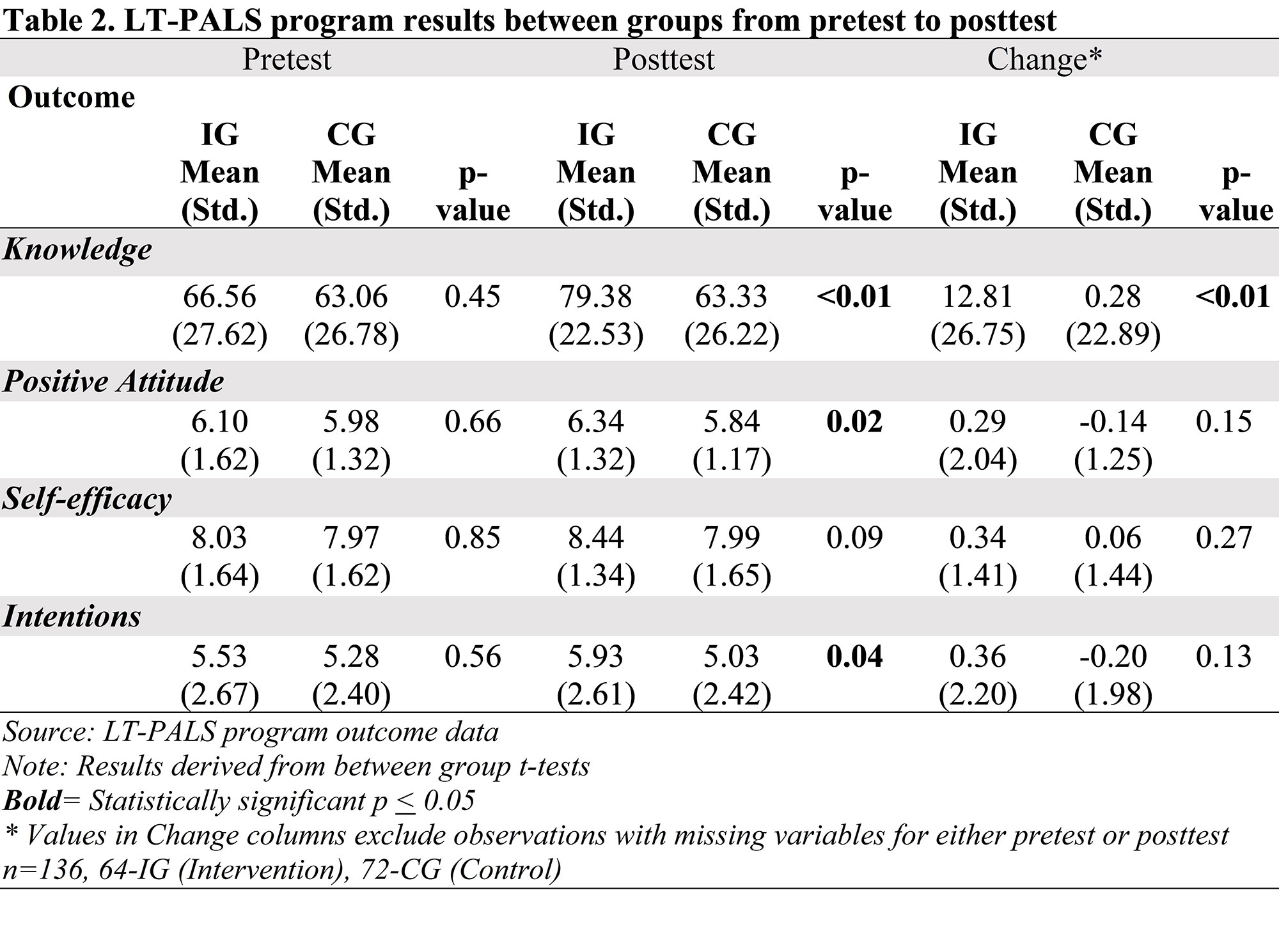
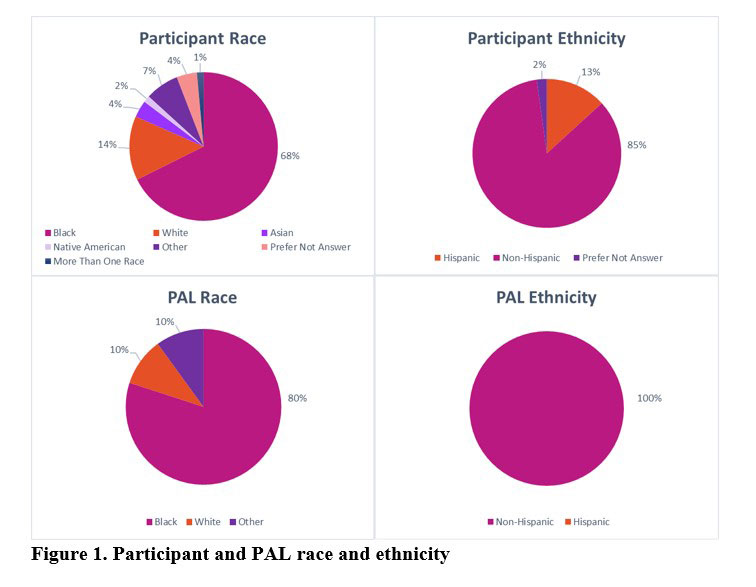
Results: The sample (n=136) included 64 in the IG and 72 in the CG, 67.7% self-identified as Black/African American. The IG had significantly higher posttest scores for Knowledge about (p< 0.01), Attitudes toward (p< 0.05), and Intentions (p< 0.05) to participate in LCTs than the CG. Regression models controlled for participant characteristics and showed significantly higher posttest scores for Knowledge (p< 0.001) and Intentions (p< 0.05) to participate in LCTs for the IG. From pretest to 3-month follow-up, none of the outcomes decreased significantly and IG Self-Efficacy scores increased significantly (p< 0.01). Intentions was the only outcome that decreased significantly (p< 0.05) from posttest to 3-month follow-up. The IG reported favorable opinions of the LT-PALS program. Black/African American and Hispanic participants rated significantly higher satisfaction levels compared to White (p< 0.01) and non-Hispanic (p< 0.05) participants, respectively. Sites reported various challenges related to the COVID-19 pandemic since research activities were halted at academic centers during that time.
Conclusion: Findings demonstrated feasibility of the LT-PALS program and showed its effectiveness in increasing Knowledge, Attitudes, and Intentions related to LCT participation from underrepresented groups, including those of Black ancestry. One-year follow-up data collection is ongoing.
To cite this abstract in AMA style:
Sheikh S, Donovan C, Menezes C, Roy A, Simkus A, Gross D, Askanase A, Ramsey-Goldman R, Majithia V, Wanty N, McNeill A, Holtz K, Lim S. Feasibility and Efficacy of a Peer Education Program to Improve Patient Engagement in Lupus Clinical Trials [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/feasibility-and-efficacy-of-a-peer-education-program-to-improve-patient-engagement-in-lupus-clinical-trials/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/feasibility-and-efficacy-of-a-peer-education-program-to-improve-patient-engagement-in-lupus-clinical-trials/