Session Information
Date: Saturday, November 16, 2024
Title: RA – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Upadacitinib (UPA), a Janus kinase inhibitor (JAKi) with three years of market experience, has not yet been the subject of a significant number of studies conducted in a real-world setting. As with other JAKi drugs, UPA has received warnings from major regulatory agencies regarding an increased risk of cardiovascular events and cancer. The UPAreal study was designed to evaluate patient characteristics associated with better UPA persistence and the impact of risk minimization measures on UPA prescribing patterns in routine clinical practice.
Methods: Observational, retrospective, multicenter study including patients diagnosed with RA (ACR/EULAR 2010 criteria) and spondylarthritis (SpA) (psoriatic arthritis, CASPAR criteria, and axial SpA, ASAS criteria) who received UPA for at least 3 months between January 2021 and December 2023. Baseline demographic and disease characteristics and activity, changes over time in DAS28 or BASDAI, baseline cardiovascular risk (CVR) by Systemic Coronary Risk Estimation (SCORE)2, concomitant medications, UPA treatment line, and adverse event data were collected. To compare baseline CVR, RA and SpA patients who initiated treatment with a tumor necrosis factor inhibitor (TNFi) during the same period were also analyzed. Cox regression and Kaplan-Meier curves were used to analyze UPA persistence and associated factors. Chi-squared, t-Student, and U-Mann-Whitney tests were used to compare groups.
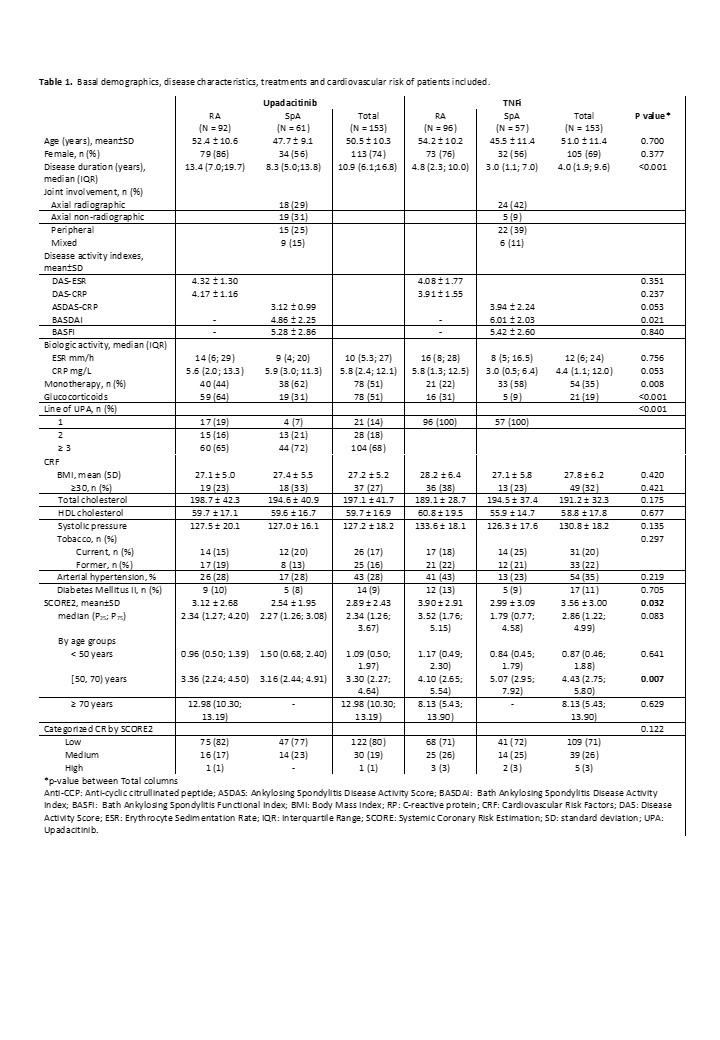
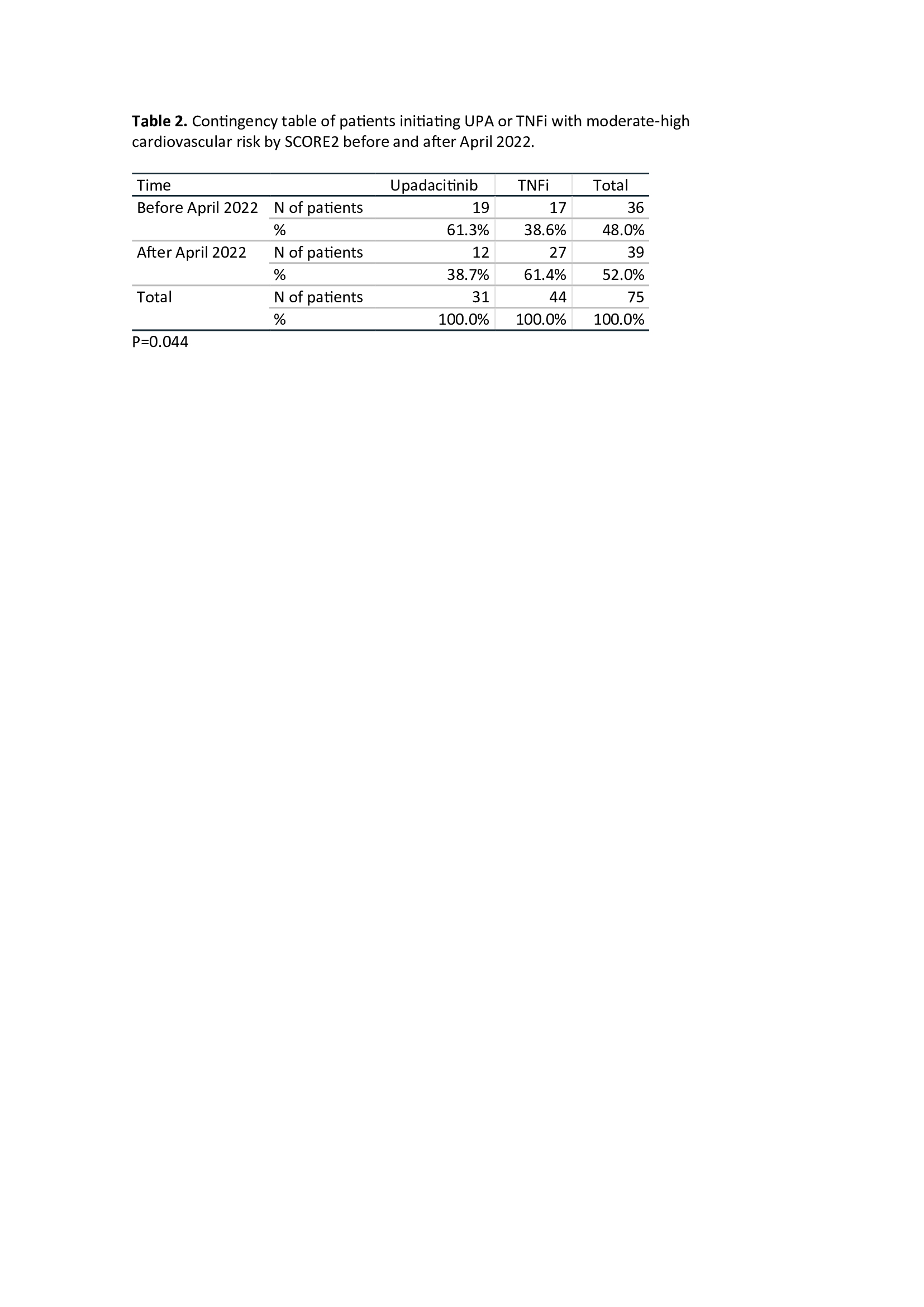
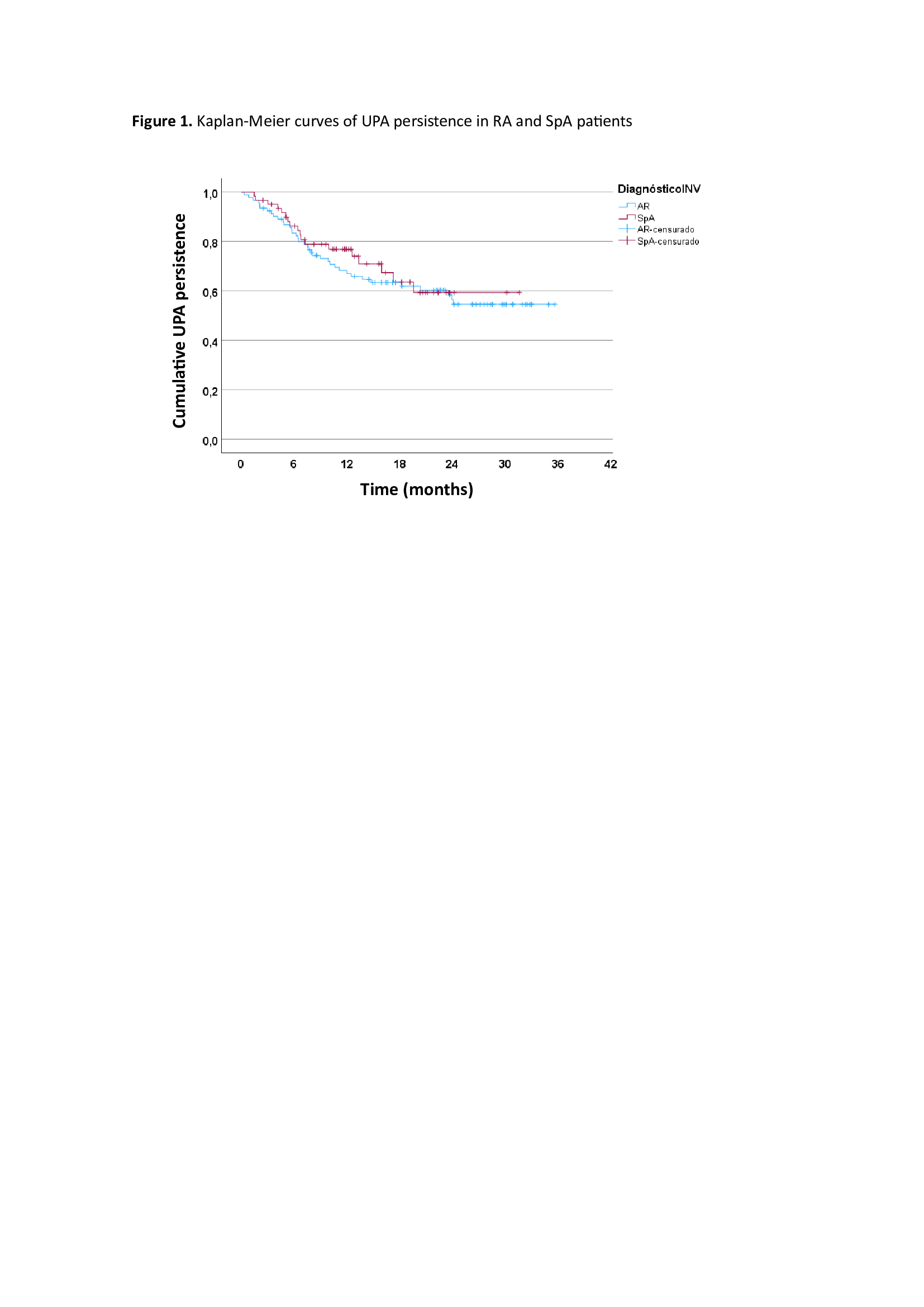
Results: A total of 306 patients were included, 153 (50%) treated with UPA and 153 (50%) treated with TNFi. Mean follow-up was 17.3±8.8 (SD) months. Table 1 shows baseline demographic and disease characteristics, disease activity and CVR factors including baseline SCORE2 of patients treated with UPA and TNFi. Basal DAS28, ASDAS and BASDAI indices were all significantly reduced at 2 years of follow-up (p< 0.001). The persistence of UPA was similar in RA and SpA patients (Figure 1), with an estimated mean persistence of 23.9±1.5 and 22.8±1.7 months, respectively. According to the Cox regression analysis including the variables with p-value < 0.2 in the univariate analysis, i.e. disease duration, treatment line and previous use of JAKi or interleukin-6 inhibitors (IL6i), the only factor associated with lower UPA persistence was the use of IL6i (HR: 2.05 95%CI 1.2-3.49, p= 0.008). The main reasons for discontinuation of UPA were ineffectiveness, 29 (52.7%); adverse events, 23 (41.8%) (infectious diseases, 15, 65%); and loss to follow-up, 3 (5.5%). With respect to CVR, baseline CVR as categorized by SCORE2 was similar between UPA- and TNFi-treated patients (p=0.122). However, when SCORE2 was categorized and analyzed by trimester, a significant reduction was observed in moderate-high risk patients initiating UPA versus TNFi since April 2022 (Table 2) (p=0.04).
Conclusion: In real-world settings, UPA behaves similarly to that observed in randomized controlled trials. Notably, the persistence of UPA is significantly lower in RA patients previously treated with IL6i. Regulatory recommendations to minimize CVR in patients treated with JAKi have been adopted and even anticipated in clinical practice.
To cite this abstract in AMA style:
Garcia Dorta A, Rodríguez-Regalado C, Hernández-Martín A, Peña-Montelongo S, Martínez-González C, Naveda-González E, González-Dávila E, Diaz-González F, Hernández-Hernández V. Factors Associated with Upadacitinib Persistence and Risk Minimization Measures in Patients with Rheumatic Diseases in a Real-world Setting. UPAreal Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/factors-associated-with-upadacitinib-persistence-and-risk-minimization-measures-in-patients-with-rheumatic-diseases-in-a-real-world-setting-upareal-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/factors-associated-with-upadacitinib-persistence-and-risk-minimization-measures-in-patients-with-rheumatic-diseases-in-a-real-world-setting-upareal-study/