Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Clinicians are encouraged to consider patient preferences for offering face-to-face vs virtual consultations. This adaptability is particularly important to rheumatologists caring for patients with long-term conditions, in whom personalized care can require ongoing adaptation. Besides advancement in technology, the relevance of remote access in rheumatology has been explored since COVID-19 pandemic. However, to date, no study has investigated remote access influence on patients’ perception on taking part in rheumatology clinical research. Herein, we present the results of patients’ perceptions with a rheumatic disease on involvement of clinical research virtually.
Methods: We conducted an online survey of patients with at least one confirmed diagnosis of a rheumatic condition attending rheumatology clinic in the UK. Questions were designed by rheumatologist with input from UK-based patient charities and four expert patients. The survey was kept open for a period of one month in 2020. Descriptive statistics were used to summarize results.
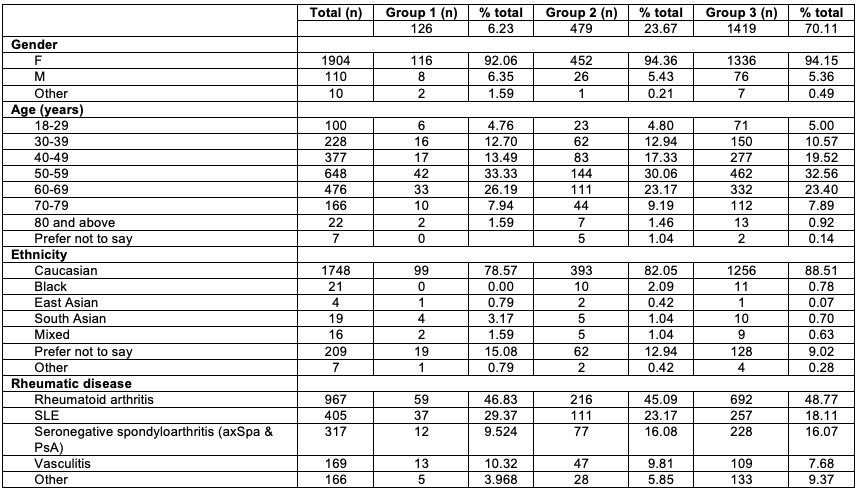
Results: Overall, 2,024 patients completed the survey. 94% were female and the majority were 50-59 years old (32%) and 86.4% were Caucasian. 209 patients (10.3%) preferred not to disclose their ethnicity. Rheumatoid arthritis (RA) was the most common diagnosis (n=967, 47.8%) followed by systemic lupus erythematosus (SLE; n=405, 20.0%). Demographic data are shown in Table 1.
Participants were categorized according to their response regarding future engagement with clinical trial following the COVID-19 pandemic: Group 1= “I will only take part in research studies that can be done by telephone or video consultation” ; Group 2= “I will take part in research studies but will not want to make additional visits to hospital for research blood tests” ; Group 3= “I am just as likely to take part in any research study offered even if it requires additional visits to hospital”. 6% (n=126) were in Group 1; 23% (n=479) in Group 2; and 69% (n=1419) in Group 3. Approximately the same proportion of females and age ranges were present across all 3 groups.
When looked specifically between ethnicity and willingness to participate in research, Caucasian patients were most likely to patriciate in future research even it required additional visits to hospital (n=1256, 88.51%) and East Asians were least likely to do so (n=1, 0.07). Those patients who preferred not to disclose their ethnicity, were most likely to choose telephone participation only (n=19, 15.8%).
Regarding specific rheumatic diseases, patients with SLE or vasculitis were most likely to select telephone participation only (Group 1), while those with inflammatory arthritis (i.e. RA and seronegative arthritis) were more likely to select face to face research participation even if additional research were required.
Conclusion: To our knowledge, this is the largest survey database exploring attitudes towards participation in future rheumatology clinical trials in the UK. We note marked differences between patients of different ethnicities and diagnosis in preferred level of engagement. Our results highlight the need to implement strategies to widen participation in traditionally understudied population.
To cite this abstract in AMA style:
Kouranloo K, Wincup C. Factors Associated with Participation in Rheumatology Clinical Trials: A UK-based Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/factors-associated-with-participation-in-rheumatology-clinical-trials-a-uk-based-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/factors-associated-with-participation-in-rheumatology-clinical-trials-a-uk-based-study/