Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Accrual of irreversible organ damage is a major risk factor for death in SLE. While unmodifiable factors, such as age at disease onset and ethnicity, are associated with increased damage accrual in SLE, disease activity and medications, in particular glucocorticoids, are also associated. Understanding the independent contribution of glucocorticoid use to organ damage in SLE is confounded by the fact that glucocorticoid use is associated with active disease. We determined factors contributing to the risk of damage accrual in SLE independent of disease activity by studying patients who had no measurable disease activity.
Methods: SLE patients were prospectively recruited from 13 centres in 8 countries and followed longitudinally between 2013-2016. Disease activity (SLEDAI-2K) and treatment details were recorded at each visit, and organ damage measured annually (SDI). Cox-proportional hazards analyses were used to examine time-dependent associations of variables with damage accrual. The absence of disease activity was defined as time-adjusted mean (TAM)-SLEDAI-2K=0.
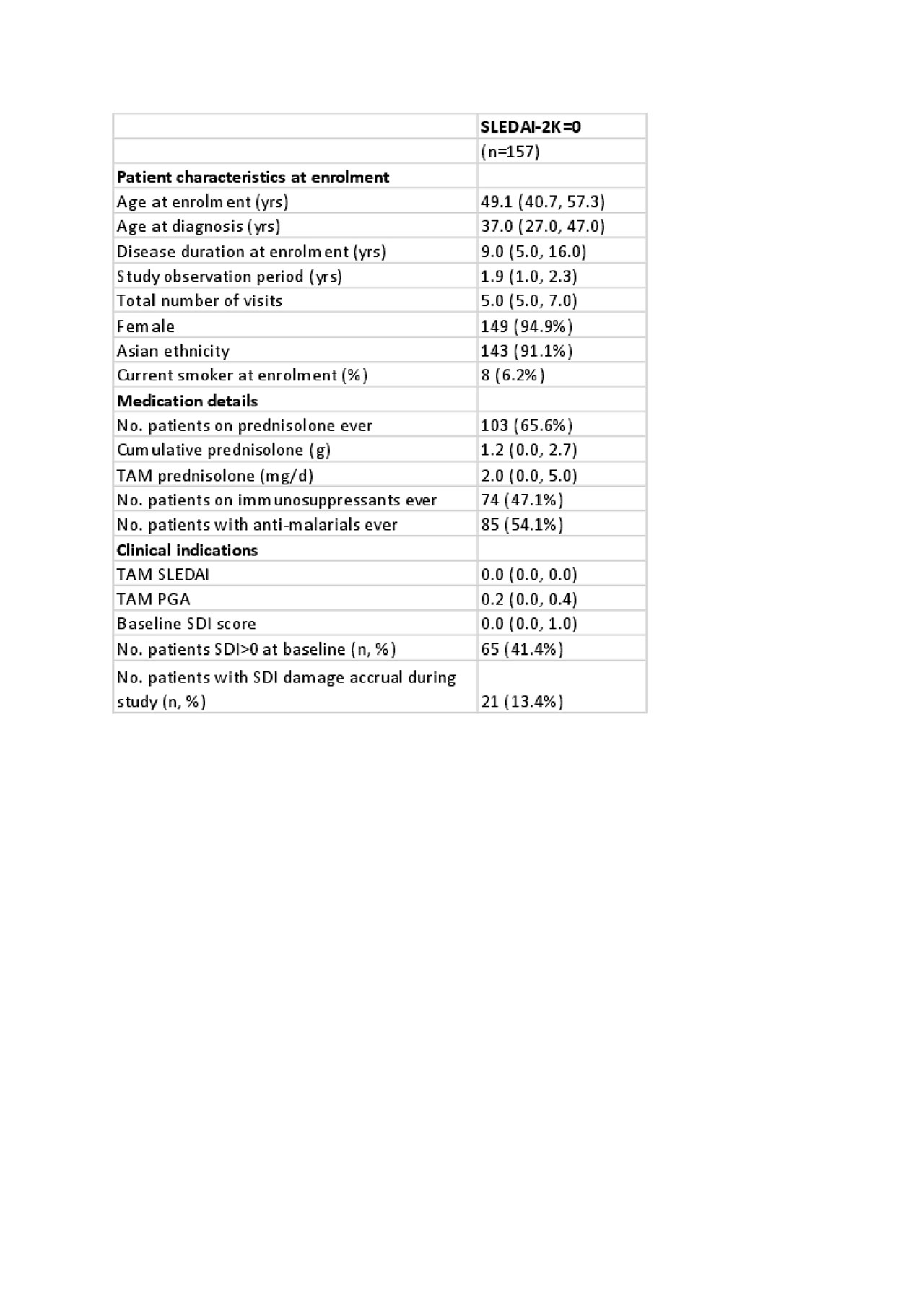
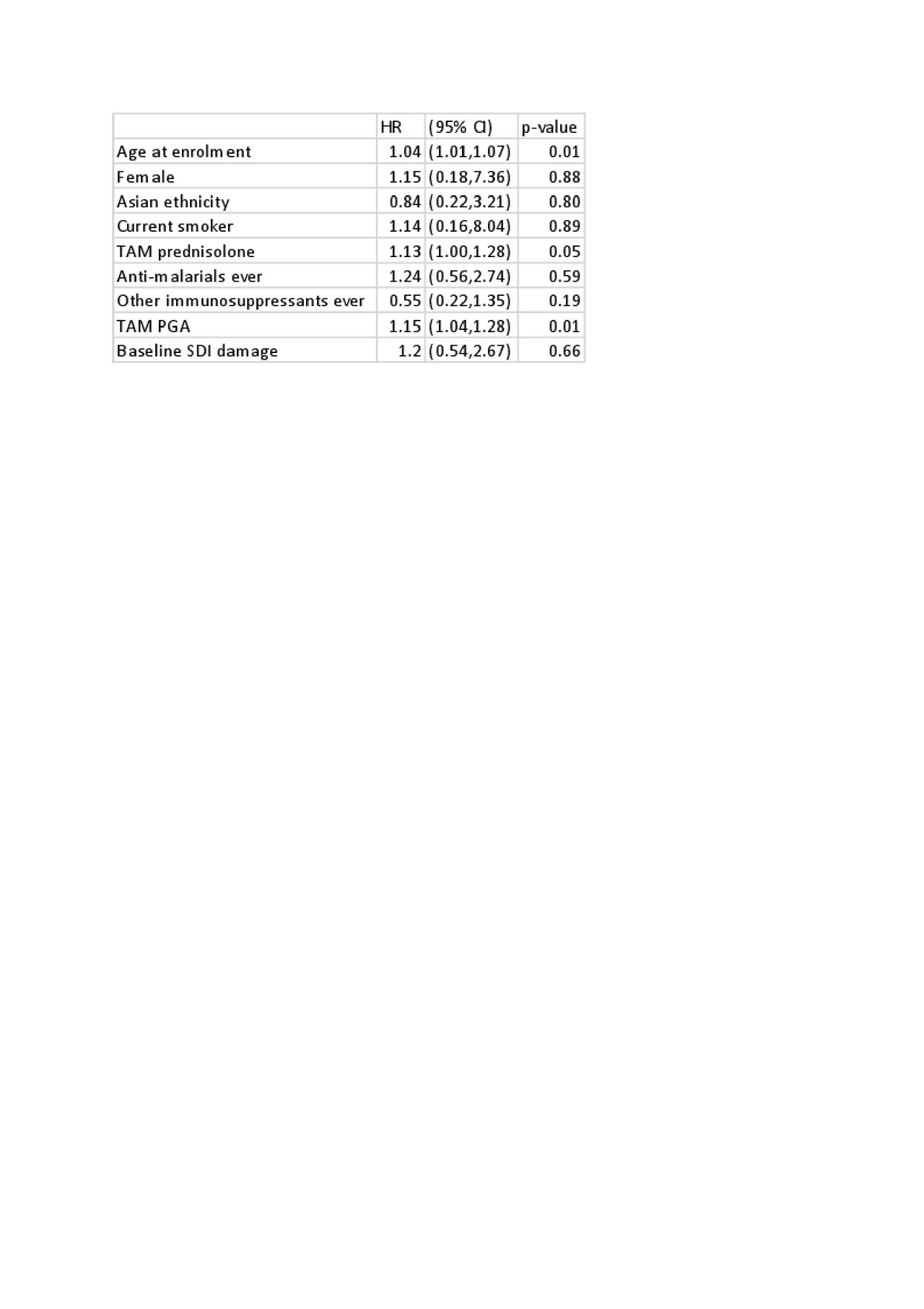
Results: 1707 patients were studied. Patient characteristics are shown in Table 1. 157 patients (9%) had no clinical or serological disease activity for the entire study period (TAM-SLEDAI-2K=0). Prednisolone exposure was 66% (103/157) in this group, with a median TAM-prednisolone dose of 2.0mg/day (0.0-5.9). Forty-one per cent (65/157) of patients had irreversible organ damage at baseline. Despite SLEDAI-2K=0 throughout, accrual of irreversible organ damage occurred in 13% of this subset, with 21 events captured over a median (IQR) 1.9 (1.0-2.3) years follow-up. In univariable analysis of time-adjusted mean variables, prednisolone exposure was associated with damage accrual (HR 1.1, 95% CI 1.0-1.3, p=0.05), as was physician global assessment (PGA) (HR 1.15, 95% CI 1.0-1.3, p=0.01). Baseline SDI organ damage, gender and ethnicity were not associated with damage accrual in this subset (Table 2). In multivariable analysis, damage accrual was independently associated with prednisolone exposure (HR 1.14, 95% CI 1.03-1.25, p=0.01), physician global assessment (PGA) (HR 1.13, 95% CI 1.03-1.23, p=0.01) and age at enrolment (HR 1.04, 95% CI 1.01-1.07, p< 0.02) (Table 3).
Conclusion: Irreversible damage accrual occurs in SLE patients with no clinical or serological disease activity as captured by SLEDAI-2K. Glucocorticoid use contributes to the risk of organ damage in these patients. These findings confirm an independent contribution of glucocorticoid use to organ damage accrual in SLE.
To cite this abstract in AMA style:
Apostolopoulos D, Kandane-Rathnayake R, Louthrenoo W, Luo S, Wu Y, Lateef A, Golder V, Sockalingam S, Navarra S, Zamora L, Hamijoyo L, Katsumata Y, Harigai M, Chan M, O'Neill S, Goldblatt F, Lau C, Hoi A, Nikpour M, Morand E. Factors Associated with Damage Accrual in SLE Patients with No Clinical or Serological Disease Activity [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/factors-associated-with-damage-accrual-in-sle-patients-with-no-clinical-or-serological-disease-activity/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/factors-associated-with-damage-accrual-in-sle-patients-with-no-clinical-or-serological-disease-activity/