Session Information
Date: Sunday, November 12, 2023
Title: (0510–0542) Spondyloarthritis Including Psoriatic Arthritis – Treatment: AxSpA Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Anti-tumor necrosis factor (TNF) agents have proven to be effective in treating Ankylosing spondylitis (AS). However, a significant number of patients discontinue or switch their anti-TNF agent for various reasons. Previous studies have been limited in scope, primarily focusing on drug retention rates or utilizing cohorts with short follow-up durations. The objective of this study is to investigate the factors associated with cause-specific discontinuation of long-term anti-TNF agent use in patients with AS.
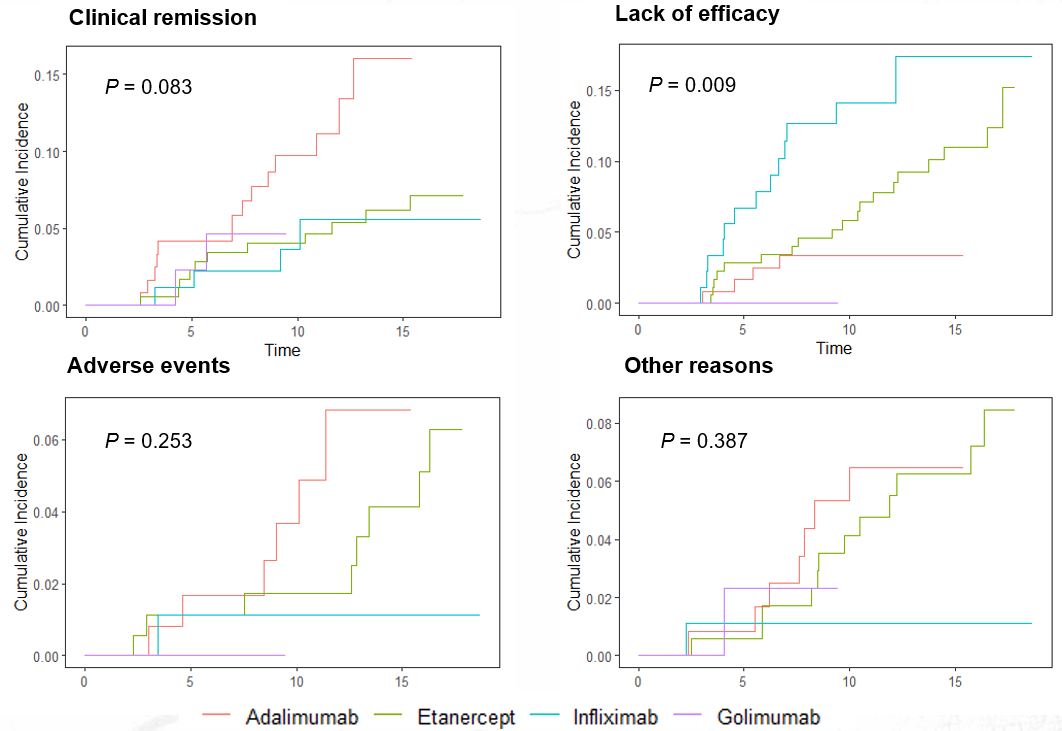
Methods: AS patients who initiated the first-line anti-TNF agent between 2004 and 2018 and continued the treatment for at least 2 years were enrolled in the study. Patients with concomitant inflammatory bowel disease were excluded. The index date was defined as the date of initiation of the first-line anti-TNF agent, and the observation period lasted until the last visit, discontinuation of the first-line anti-TNF agent, or September 2022. The reasons for discontinuation of the first-line anti-TNF agent were categorized into 1) clinical remission, 2) lack of efficacy, 3) adverse events, and 4) other reasons including loss to follow-up, cost, or reimbursement issues. The cumulative incidence function curve was used to visualize the cumulative failure rates over time for each specific reason. Univariate and multivariate cause-specific hazard models were utilized to identify factors associated with cause-specific discontinuation of the first-line anti-TNF agent.
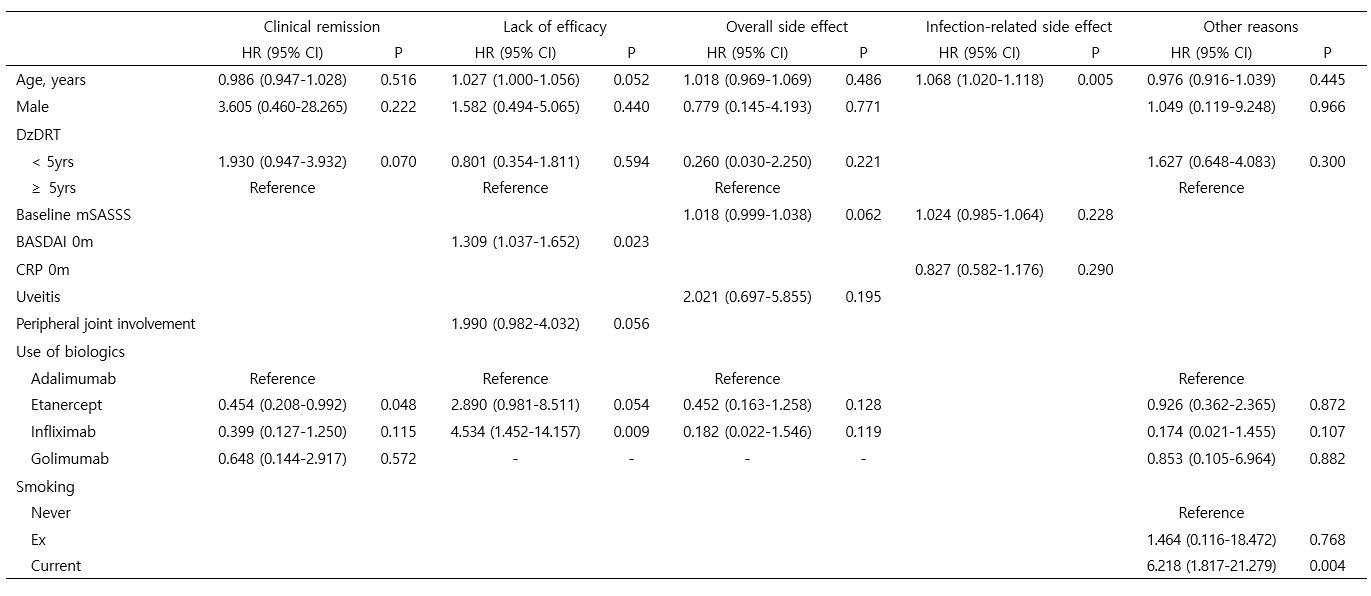
Results: A total of 429 AS patients were included in the study, with 121 on adalimumab (ADA), 176 on etanercept (ETN), 89 on infliximab (INF), and 43 on golimumab (GLM). The median overall survival on the first-line anti-TNF agent was 10.6 (7.9-14.5) years. Among the patients, 103 (24.0%) discontinued the first-line anti-TNF agent, with 36 (34.9%) due to inefficacy, 31 (30.1%) due to sustained clinical remission, 15 (14.6%) due to adverse events, and 21 (20.4%) due to other reasons. Patients treated with ETN had a lower risk of discontinuation due to sustained clinical remission compared to those on ADA (Hazard ratio [HR] 0.45 [0.21-0.99], P=0.048). Higher baseline BASDAI (HR 1.31 [1.04-1.65], P=0.023) and use of INF were associated with a higher likelihood of discontinuation due to inefficacy compared to ADA (HR 4.53 [1.45-14.16], P=0.009). Older age was related to an increased risk of discontinuation due to infection-related adverse events (HR 1.07 [1.02-1.12], P=0.005), and current smoking was a risk factor for discontinuation due to other reasons (HR 6.22 [1.82-21.28], P=0.004).
Conclusion: Several factors influencing cause-specific discontinuation of long-term anti-TNF treatment were identified in our study. Further investigation is needed to understand the underlying reasons for these phenomena. This knowledge is valuable for improving personalized medicine in the management of patients with AS, as it allows for more informed decision-making and the customization of treatment plans based on individual patient characteristics.
To cite this abstract in AMA style:
nam b, Choi N, Koo B, Kim J, Shin J, lee S, Joo k, Kim T. Factors Associated with Cause-specific Discontinuation of Long-term Anti-tumor Necrosis Factor Agent Use in Patients with Ankylosing Spondylitis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/factors-associated-with-cause-specific-discontinuation-of-long-term-anti-tumor-necrosis-factor-agent-use-in-patients-with-ankylosing-spondylitis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/factors-associated-with-cause-specific-discontinuation-of-long-term-anti-tumor-necrosis-factor-agent-use-in-patients-with-ankylosing-spondylitis/