Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Biologic disease modifying agents (DMARDs) are an integral part of rheumatoid arthritis (RA) treatment, but the adoption of infusion-based products has not been reported. We examined Medicare claims to determine the relative market shares for biologic agents in beneficiaries with RA.
Methods: We identified Medicare enrollees with a least one diagnosis code from an inpatient or outpatient claim for RA between 2012-2016. We pulled their Medicare Part B claims (20% Part B sample) for biologic infusions using J-codes. We aggregated procedure claims to the facility level for facilities with at least 50 claims and characterized the distribution of individual biologic agents annually. We report trends in the adoption and reductions in use of agents overall and according to facility size.
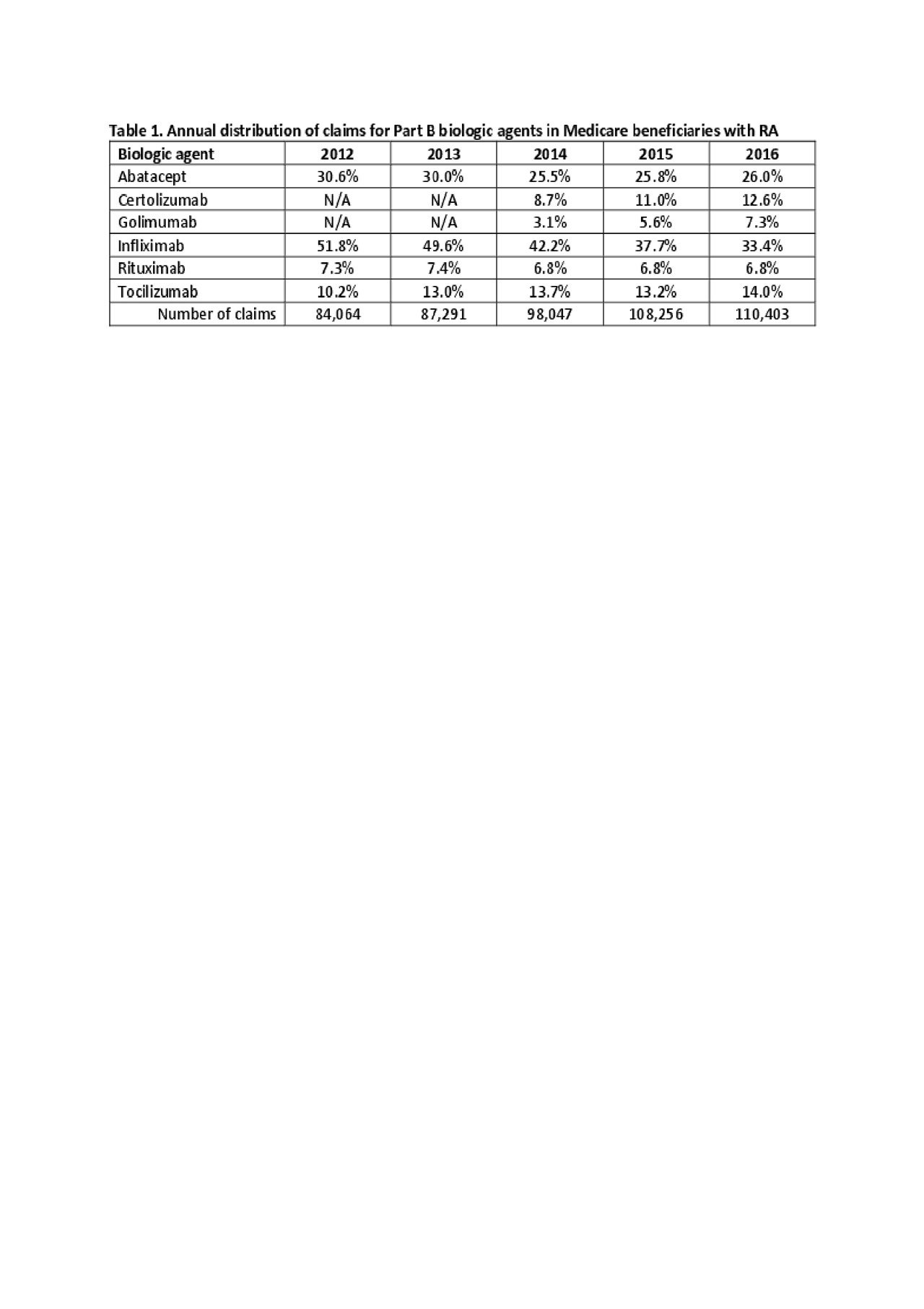
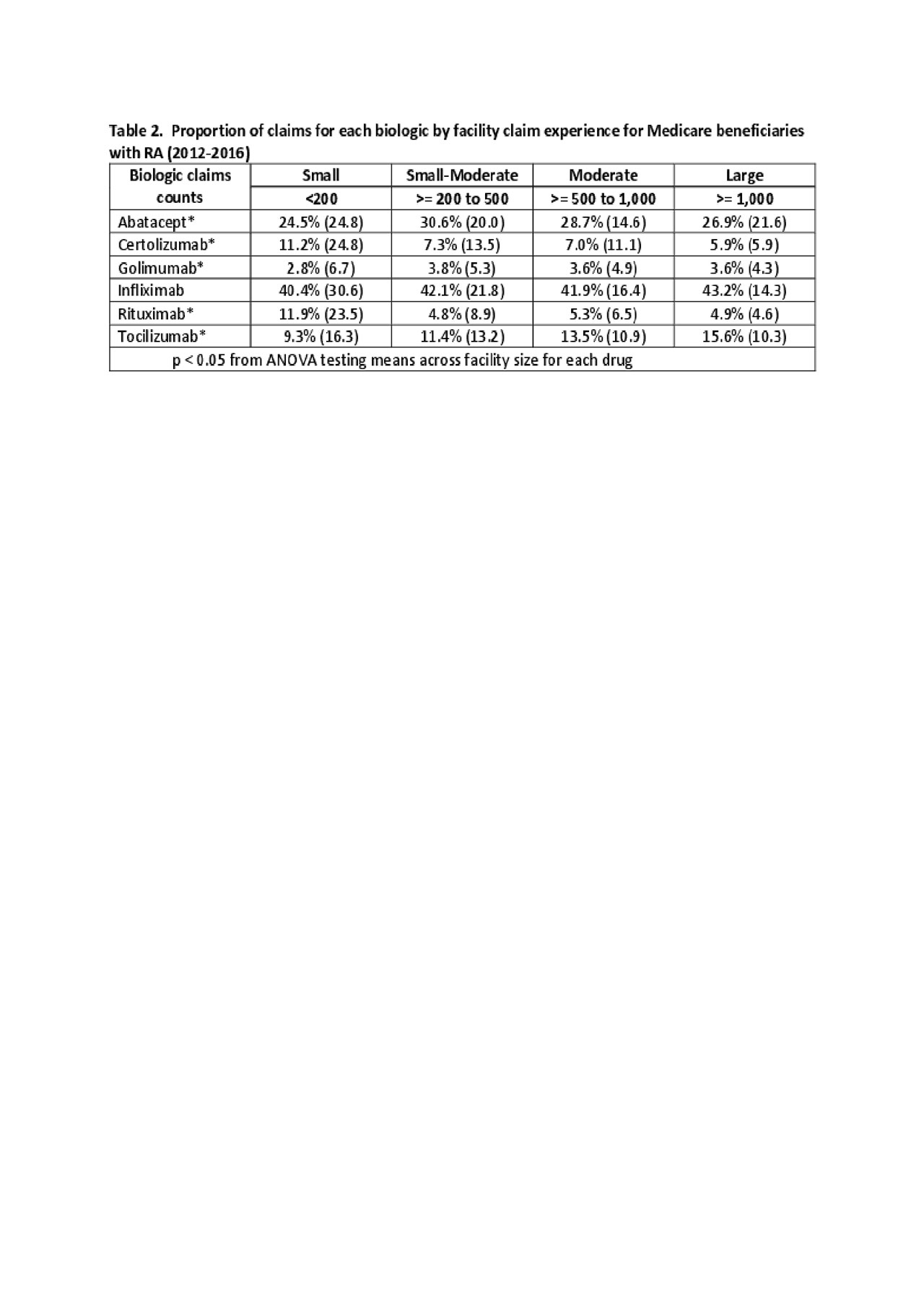
Results: There were 27,143 Medicare enrollees with RA: mean age, 68.8 years ± 10.1; white, 86.6%, and black, 7.4%; female, 76.8%. Part B claims for biologics increased 31.3%: Infliximab was most common, though its use dropped from 51.8% to 33.4%; abatacept dropped from 30.6% to 26.0% (Table 1). Tocilizumab, certolizumab and golimumab increased use over time, and rituximab remained consistent (6.8%-7.4%). Biologic use varied significantly by facility size for all agents except infliximab (Table 2). Smaller facilities used more certolizumab (11.2%) and rituximab (11.9%).
Conclusion: Office administration of biologic agents for RA has expanded by nearly one-third in 5 years. Infliximab and abatacept lost market share as newer agents were introduced; total share anti-TNF agent remained constant. Further research is needed to determine how facilities select between agents and the impact on patient outcomes.
To cite this abstract in AMA style:
Dalal D, Zhang T, Verma H, Shireman T. Facility-Level Variation in Biologic Disease Modifying Agents for Medicare Enrollees with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/facility-level-variation-in-biologic-disease-modifying-agents-for-medicare-enrollees-with-rheumatoid-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/facility-level-variation-in-biologic-disease-modifying-agents-for-medicare-enrollees-with-rheumatoid-arthritis/