Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Treatment of pain remains a priority for patients (pts) with RA. Otilimab (GSK3196165) is a human mAb that inhibits GM-CSF, a key driver in a broad range of immune-mediated conditions that is involved in the regulation of musculoskeletal pain. In the Phase 2b dose-ranging BAROQUE study (201755)1 in pts with moderate to severe RA who failed MTX, otilimab plus MTX provided rapid and substantial improvement from baseline (BL) in pain vs placebo (PBO) as assessed by a 100 mm Visual Analogue Scale (VAS). The objective of this post hoc analysis was to further define the clinical relevance of the effect of otilimab on pain through assessment of responder rates.
Methods: BAROQUE was a Phase 2b multicenter, PBO-controlled study in 222 pts with RA (ACR 2010 criteria). Pts were randomized (1:1:1:1:1:1) to subcutaneous PBO or otilimab (22.5, 45, 90, 135, or 180 mg) plus MTX once weekly for 5 weeks, then every other week until Week 50. Pts without a good/moderate EULAR response at Week 12 or with DAS28(CRP) >3.2 at Week 24 escaped to otilimab 180 mg until study end. Change from BL in pain was assessed using a 100 mm VAS. Percentage of pts with pain improvement ≥minimal clinically important difference (MCID; 10% VAS response) and improvement in pain from BL of ≥30%, ≥50%, and ≥70% were compared by treatment groups at each visit up to Week 12. The analysis was restricted to pts with complete information; missing data or dropouts prior to Week 12 were not imputed.
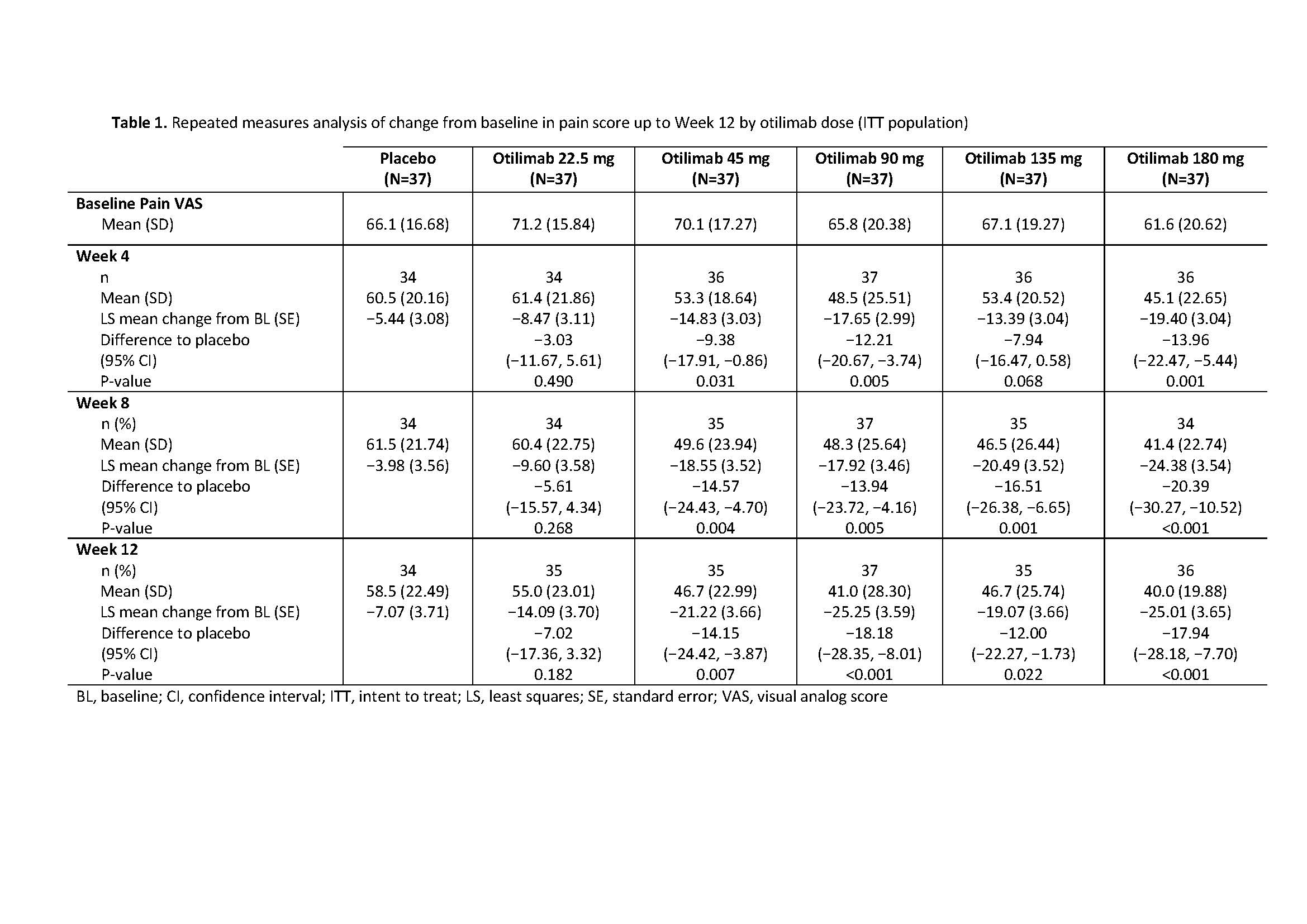
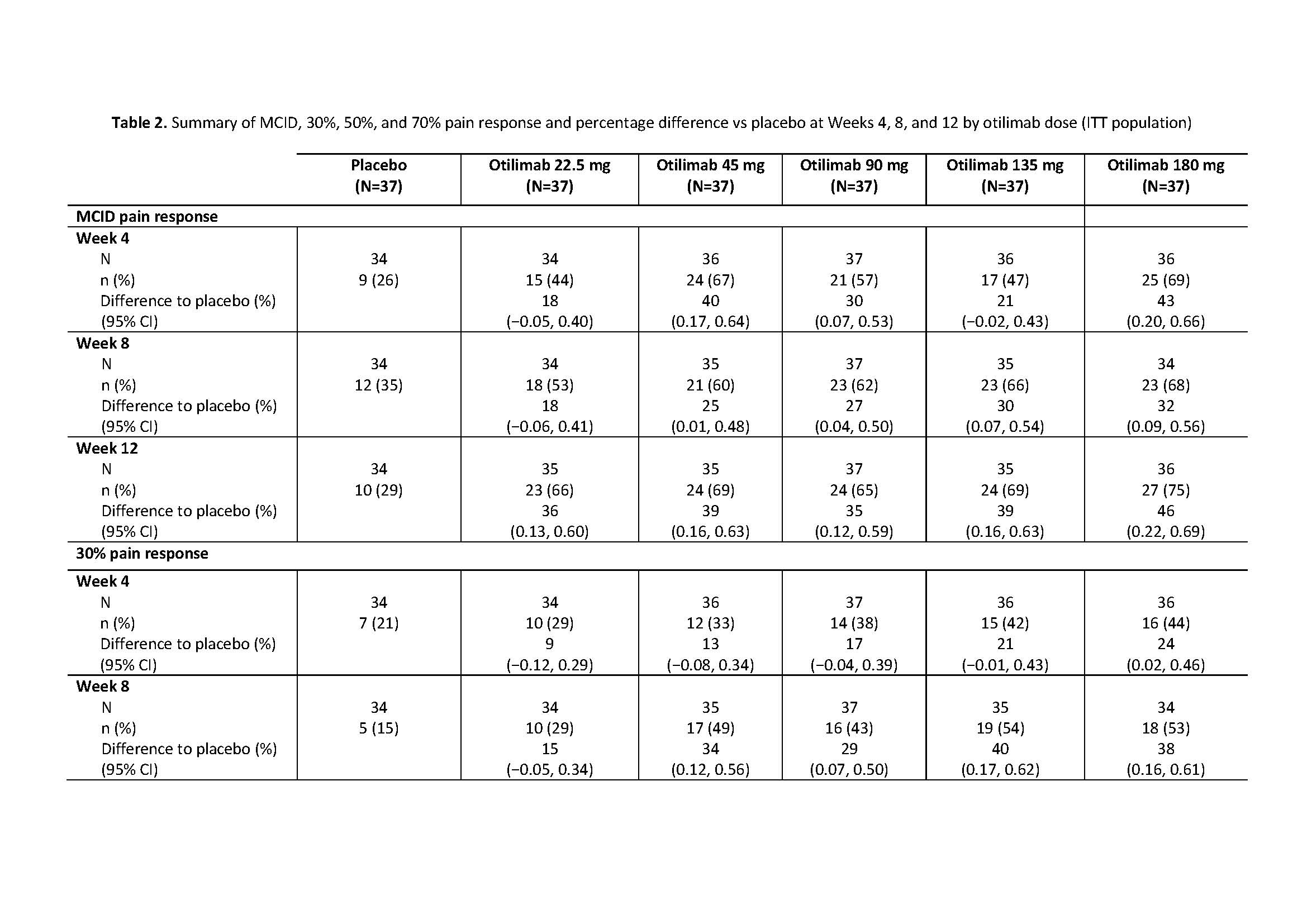
Results: The BAROQUE study population had high baseline pain VAS (mean 67.0; standard deviation 18.49). Change from BL in pain VAS is shown in Table 1. Overall, otilimab dose groups showed a higher proportion of pts with pain improvement ≥MCID vs PBO, with the largest difference vs PBO in the 180 mg dose group at Week 12: 46% (95% CI 22%, 69% [75% vs 29% of pts with MCID]) (Table 2). At most timepoints, more pts achieved 30%, 50%, and 70% pain responses in the otilimab groups vs PBO and the difference generally increased with increasing treatment duration (Table 2). At Week 12 in the 90−180 mg dose groups, the difference vs PBO in 30% pain response ranged from 25% to 43% (greatest difference with otilimab 180 mg), 50% pain response ranged from 19% to 26% (greatest difference with otilimab 90 mg), and 70% pain response ranged from 5% to 21% (greatest difference with otilimab 90 mg).
Conclusion: Further to the observed clinically relevant change from baseline in VAS pain with otilimab plus MTX in the overall BAROQUE study population, this post hoc analysis shows that a greater number of pts had substantial (≥50%) improvement from BL in pain levels when treated with otilimab plus MTX vs PBO over 12 weeks. Phase 3 studies are ongoing in pts with moderate to severe active RA and will further assess the overall clinical benefit and sustained improvement in pain symptoms with weekly dosing of otilimab plus MTX.
Study funded by GSK (NCT02504671); GSK involved in design and data analysis. Medical writing support was provided by Olga Conn, PhD, Fishawack Indicia Ltd, UK, funded by GSK.
1. Buckley C et al. ACR 2018; abstract 1938 (oral)
BAROQUE Pain ACR 2019 abstract_Table 1
BAROQUE Pain ACR 2019 abstract_Table 2
To cite this abstract in AMA style:
Buckley C, Campos J, Zhdan V, Becker B, Collignon O, Hawkes C, Layton M, Patel J, Davy K, Gupta A, Fernandes S. Exploratory Analysis of a Phase 2b Study Confirms Substantial Pain Improvement with Anti-GM-CSF Monoclonal Antibody Otilimab (GSK3196165) in Patients (Pts) with Active RA [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/exploratory-analysis-of-a-phase-2b-study-confirms-substantial-pain-improvement-with-anti-gm-csf-monoclonal-antibody-otilimab-gsk3196165-in-patients-pts-with-active-ra/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/exploratory-analysis-of-a-phase-2b-study-confirms-substantial-pain-improvement-with-anti-gm-csf-monoclonal-antibody-otilimab-gsk3196165-in-patients-pts-with-active-ra/
