Session Information
Date: Monday, November 13, 2023
Title: (1412–1441) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II: SpA
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Dietary Interventions in psoriatic arthritis (DIPSA) is a randomized controlled trial (RCT) assessing the efficacy of dietary modifications in patients with psoriatic arthritis (PsA). Ideally, the design of an adjunct therapy trial and the intervention itself should engage patients in a positive experience. We aimed to describe patients’ experience participating in a dietary RCT in a rheumatology setting.
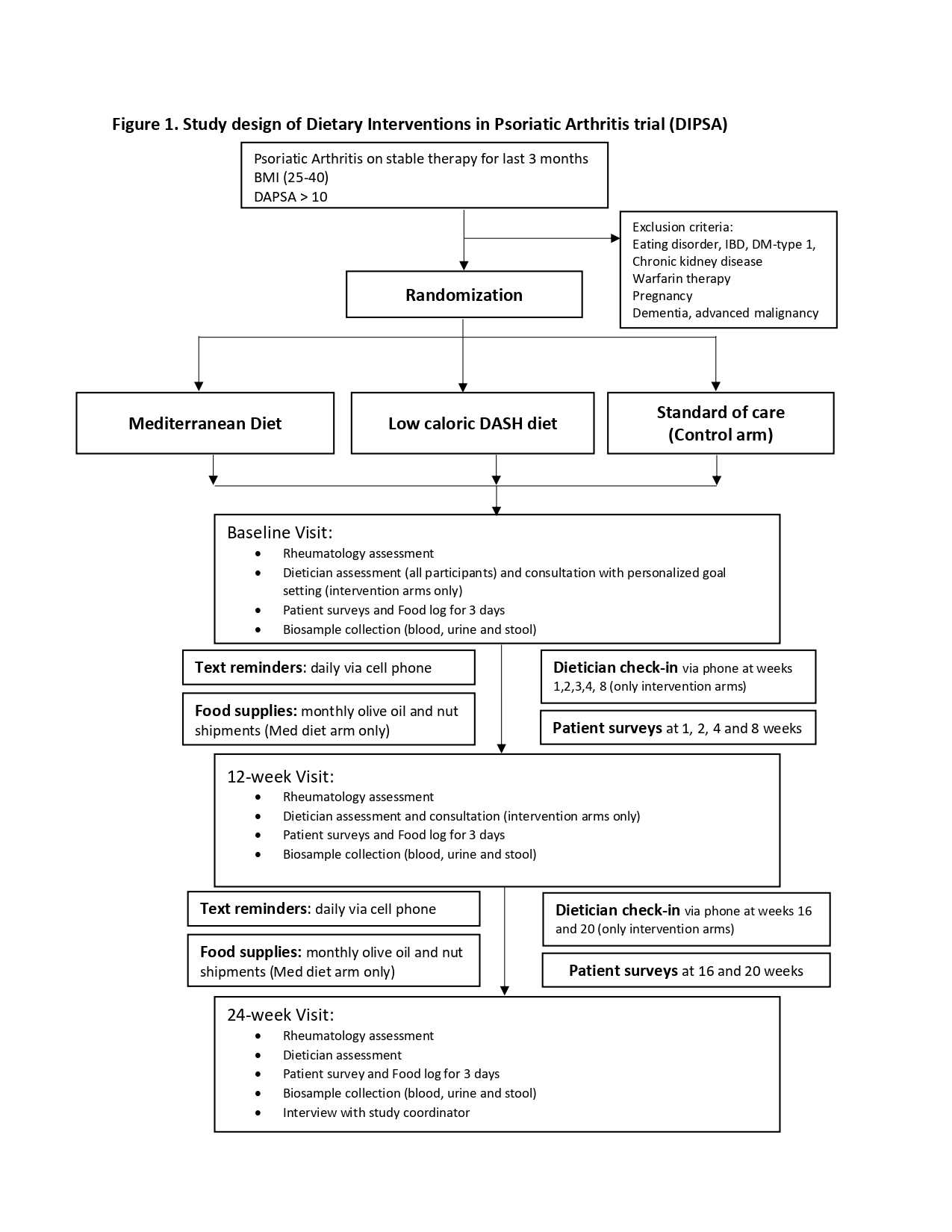
Methods: DIPSA (NCT04180904) compares the efficacy of the Mediterranean diet and DASH-low caloric diet as adjunct therapy to the standard of care in PsA. Patients with moderately active PsA (DAPSA > 10) who were overweight or obese (BMI 25-40) were enrolled into the trial for 24 weeks. The trial design is shown in Figure 1. At the end of the trial, participants underwent a semi-structured interview regarding their participation including challenges faced, positive aspects of the trial, and recommendations for a future intervention and/or trial. Interviews lasted 20-30 min and were carried out by trained research coordinators. The interviews of first 50 participants who completed the trial (out of intended 90) were analyzed using modified grounded theory by two research coordinators with input from the two PIs.
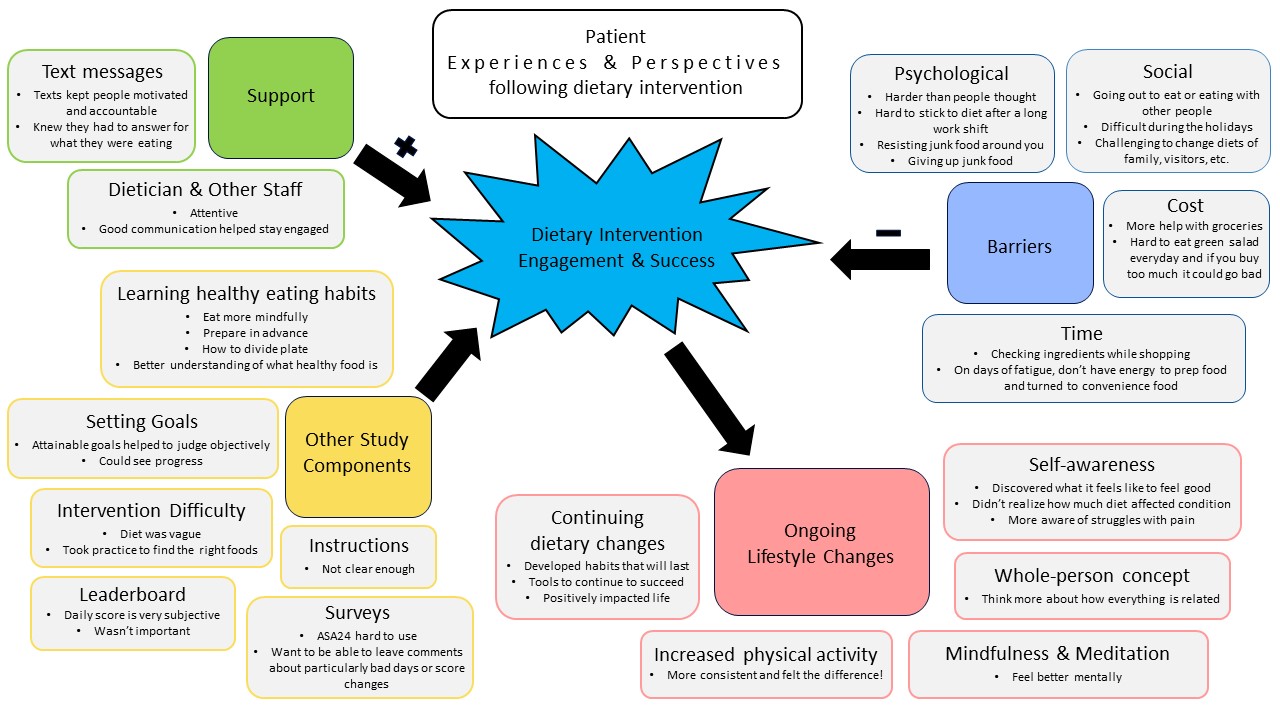
Results: The mean age of the participants was 54.8 years (SD 12.3 years) and 66% were females. Key themes identified included the benefit of increased wellness from healthy diet, structured intervention, support from dietitian and other staff, self-awareness, barriers to dietary changes, and other lifestyle changes (Figure 2). The themes, subthemes and a few representative quotes are included in Table 1. Patients found advice regarding healthy eating habits, setting personal goals, incorporating or avoiding particular food items in the diet to be beneficial for their overall health and well-being. However, most wished for a well-organized and structured diet in the form of recipes, menus, or a booklet. Support from the staff, phone calls from a dietitian and daily text messages motivated participants to adhere to the diet. Participants became more cognizant of the effect of diet on their pain and arthritis which led to higher confidence in controlling pain through dietary modification. Our study identified some important barriers to dietary change in PsA. Psychological barriers included reluctance to change the diet because of taste and habit, and difficulty adhering during times of stress and fatigue. Social issues arose when eating out, traveling or dining with others. Other barriers to healthy eating were related to time constraints and affordability. Finally, participants expressed a desire to continue beneficial dietary changes as well as to commit to other healthy lifestyle choices, e.g., increased physical activity, mindfulness and meditation.
Conclusion: Patients with PsA found the DIPSA interventions to be feasible and valuable. Support from a multidisciplinary team, especially dietitian advice, was deemed crucial. Psychosocial barriers, cost and time constraints prevent patients from implementing healthy dietary changes. Finally, focusing on dietary change had carryover, helping patients to make healthy choices in other aspects of life.
To cite this abstract in AMA style:
Eder L, Tarannum S, Bush K, Cohen S, Compher C, Emanoilidis H, Gillespie S, Gladman D, Chandran V, Scher J, Ogdie A. Experiences and Perspectives of Patients with Psoriatic Arthritis Participating in a Randomized Controlled Trial of Dietary Interventions [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/experiences-and-perspectives-of-patients-with-psoriatic-arthritis-participating-in-a-randomized-controlled-trial-of-dietary-interventions/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/experiences-and-perspectives-of-patients-with-psoriatic-arthritis-participating-in-a-randomized-controlled-trial-of-dietary-interventions/