Session Information
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Baricitinib (bari), an oral selective Janus kinase (JAK) 1/2 inhibitor, has been approved in the EU for the treatment of adults with moderately to severely active RA. We compared the in vitro cellular pharmacology of bari to upadacitinib (ABT), filgotinib (filgo), and tofacitinib (tofa), three JAK inhibitors (JAKis) currently approved or in clinical development.
Methods: Peripheral blood mononuclear cells from healthy donors (n=6-12) were incubated with different JAKis over a 7-point concentration range. Following cytokine stimulation, levels of phosphorylated signal transducer and activator of transcription (pSTAT) were measured and IC50 calculated in phenotypically gated leukocyte subpopulations. Therapeutic dose relevance of the in vitro analysis was assessed using calculated mean concentration-time (CT) profiles over 24 h obtained from JAKi-treated subjects (bari 4 mg QD; ABT 15 & 30 mg QD; filgo 100 & 200 mg QD; tofa 5 & 10 mg BID). Time above IC50 (T>IC; h/day) and average daily % inhibition of pSTAT formation (%SI) were calculated for each JAKi, cytokine, and cell type.
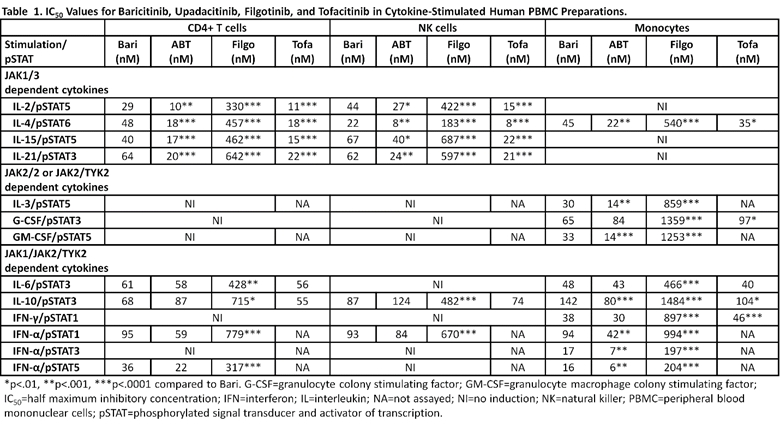
Results: The cytokines tested did not signal in all cell types (Table 1). When signaling was detected, IC50, %SI, and T>IC for a particular JAKi were similar across cell types and exhibited dose dependent inhibition (Tables 1 & 2). For JAK1/3 dependent signaling across 4 cytokines (IL-2, 4, 15, 21), IC50 for ABT and tofa were more potent than bari; filgo was the least potent. Overlaid on CT profiles, these results indicated generally higher %SI and longer T>IC for ABT and tofa compared to bari and filgo. For IL-6 (JAK1/2), %SI and T>IC was tofa > bari/ABT > filgo and for IL-10 (JAK1/TYK2), %SI was tofa > bari/ABT > filgo. IFN-γ (JAK1/2) was modulated by bari, ABT, and tofa, but not by filgo. IFN-α (JAK1/TYK2) signaling was most potently inhibited with bari and ABT, and less with filgo. Filgo did not appear to modulate GM-CSF signaling (JAK2/2), while %SI and T>IC were similar between bari and ABT.
Conclusion: JAKis display different in vitro pharmacologic profiles which, coupled to their in vivo pharmacokinetics, suggest that they modulate distinct cytokine pathways to differing degrees and durations over 24 h. Bari and filgo inhibited JAK1/3 signaling to a lesser extent than ABT and tofa. Consistent across JAKis, no agent potently or continuously inhibited an individual cytokine signaling pathway throughout the dosing interval as assessed by %SI or T>IC, respectively. These observations may have implications for the varying efficacy and safety profiles observed with JAKis across disease states.
To cite this abstract in AMA style:
McInnes IB, Higgs R, Lee J, Macias WL, Na S, Ortmann RA, Rocha G, Wehrman T, Zhang X, Zuckerman SH, Taylor PC. Ex Vivo Comparison of Baricitinib, Upadacitinib, Filgotinib, and Tofacitinib for Cytokine Signaling in Human Leukocyte Subpopulations [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/ex-vivo-comparison-of-baricitinib-upadacitinib-filgotinib-and-tofacitinib-for-cytokine-signaling-in-human-leukocyte-subpopulations/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ex-vivo-comparison-of-baricitinib-upadacitinib-filgotinib-and-tofacitinib-for-cytokine-signaling-in-human-leukocyte-subpopulations/