Session Information
Date: Monday, November 18, 2024
Title: RA – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Methotrexate (MTX) continues to be the cornerstone therapy in the treatment of rheumatoid arthritis (RA) and is usually the first conventional synthetic (cs) DMARD to be initiated, frequently in combination with steroids. This study investigated the evolution of MTX/prednisone use over the last fifteen years in the context of the increasing availability of biologic (b) and targeted synthetic (ts) DMARDs.
Methods: Data from patients (pts) enrolled in the CorEvitas RA registry over a 15-year period were analyzed. Pts initially treated with MTX who later initiated b/ts therapy were included. Details of MTX and prednisone treatment at the initiation of the first b/ts DMARD were described. Differences were compared per 3-year periods from 2008 to 2022. In addition, time to first b/ts agent was described per year from 2006 to 2020. Cox regression analysis compared the time to biologic per year adjusting for pertinent covariates. Methotrexate and steroid continuation rates and dosages after the initiation of first b/ts DMARD was described by year.
Adjusted coefficients and odds ratios (OR) for the use of MTX / prednisone prior and after the first b/ts initiation were adjusted for pertinent covariates.
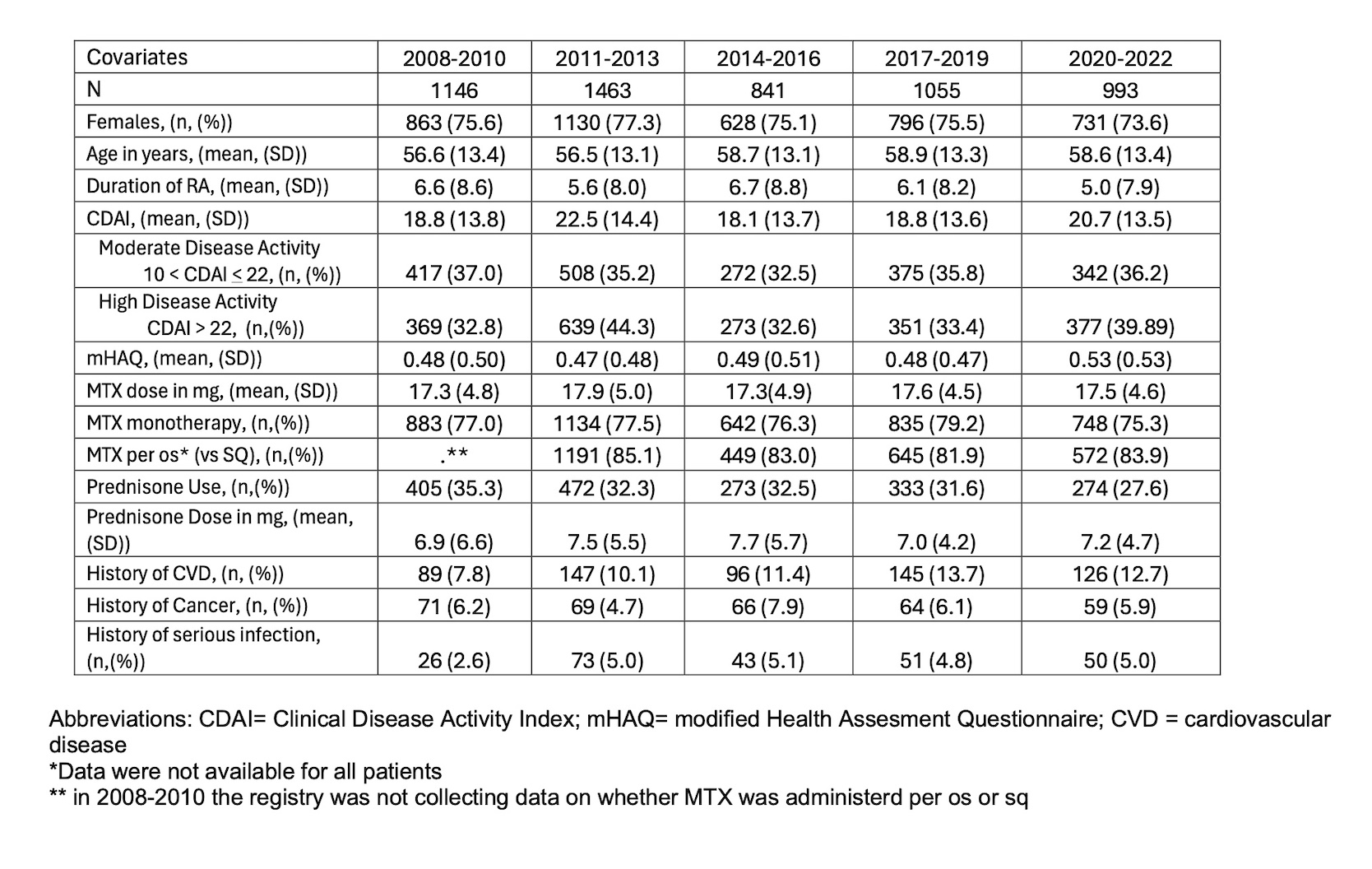
Results: The analysis included 5498 pts. Disease duration at the time of first b/ts DMARD prescription decreased in more recent years. Mean Clinical Disease Activity Index (CDAI) and the proportion of pts with moderate/high disease activity at b/ts DMARD initiation remained relatively unchanged. MTX dosage remained stable, but the rate of concurrent prednisone use decreased significantly over time (adjusted OR: 0.958 (0.010), p < 0.001). Pts on prednisone received a relatively stable mean prednisone dose over the years (6.9 to 7.7 mg). The proportion of pts on MTX in combination with other csDMARDs remained consistent. (Table 1).
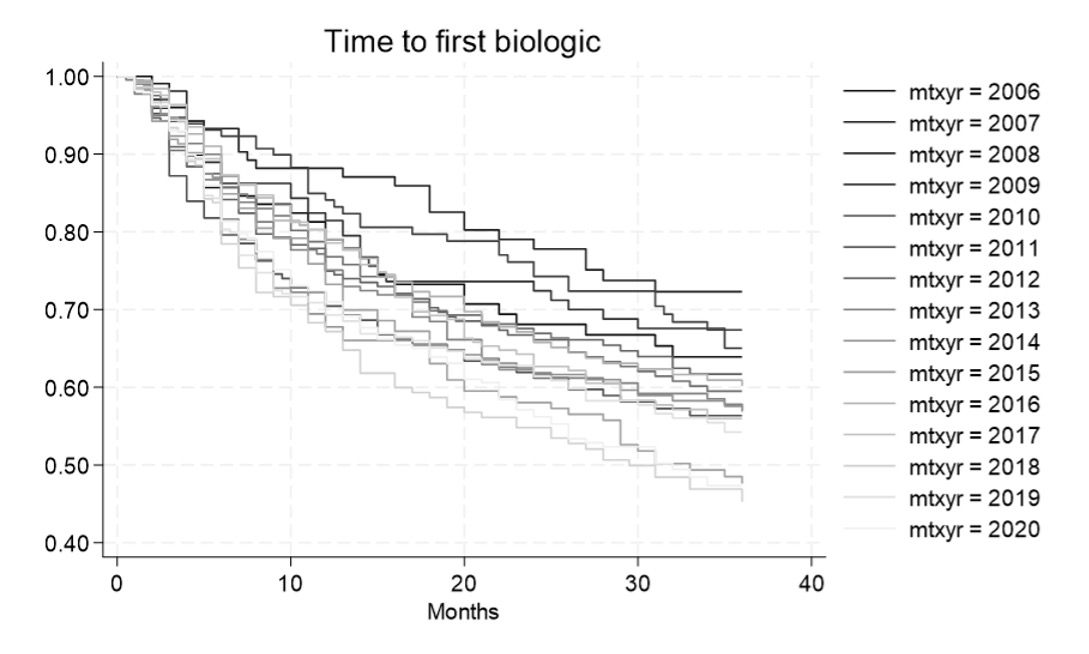
The time to the initiation of the first b/ts DMARD decreased between 2006 and 2020 (Figure 1). In earlier years (2006-2009) 25% of pts started a b/ts DMARD 22.5 months after MTX initiation; in recent years this interval decreased to 9 months. Cox regression revealed a per year Hazard Ratio of 1.03, (95% CI: 1.02 -1.05)
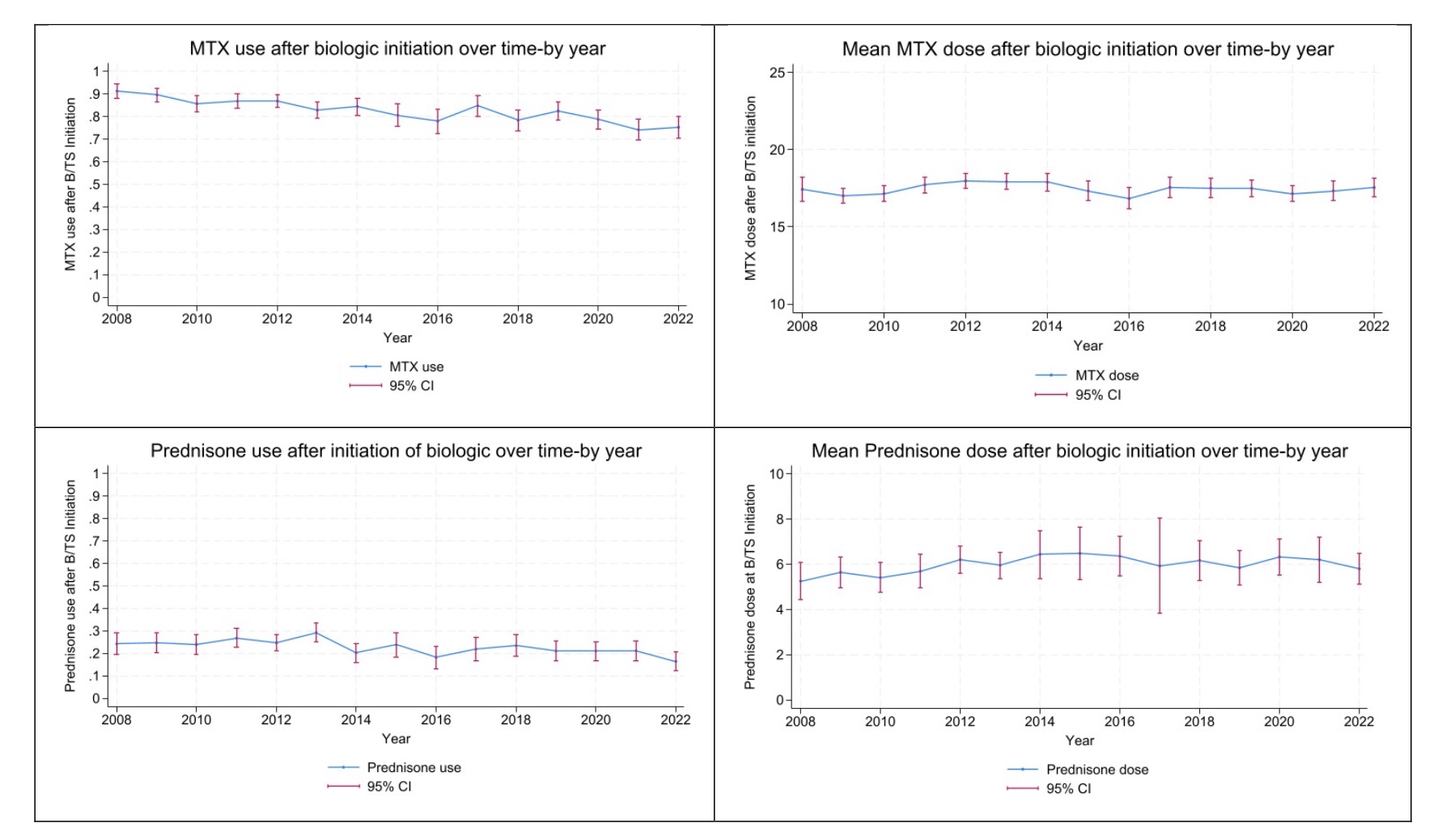
The percentage of pts continuing MTX after the initiation of first b/ts DMARD decreased over the years (adjusted OR: 0.943 (0.018), p = 0.003) but the dosage of those remaining on MTX remained stable (Figure 3). The percentage of pts continuing prednisone after b/ts DMARD initiation was lower over time (adjusted OR: 0.942 (0.015), p < 0.001). The dosage of those remaining on prednisone decreased in recent years (β= 0.264 (0.132), p = 0.045) (Figure 2).
Conclusion: The utilization of MTX in RA treatment remained consistent over the years, with stable MTX dosages and disease activity at biologic initiation. The percentage of pts on prednisone decreased over time but among users dosage remained above the recommended levels. Pts started their first b/ts DMARD earlier in their disease course and the time between MTX and first b/ts DMARD initiation gradually decreased. Statistically more patients discontinued MTX/ steroids after the prescription of b/tsDMARDs over time. Further research is warranted to determine the impact of the observed changes on pts outcomes.
To cite this abstract in AMA style:
Pappas D, Reed G, kane K, Weinblatt M, Kremer J. Evolution of Methotrexate and Prednisone Use in the Era of Biologic Therapy: 15-Year Findings from a Large Rheumatoid Arthritis Registry [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/evolution-of-methotrexate-and-prednisone-use-in-the-era-of-biologic-therapy-15-year-findings-from-a-large-rheumatoid-arthritis-registry/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evolution-of-methotrexate-and-prednisone-use-in-the-era-of-biologic-therapy-15-year-findings-from-a-large-rheumatoid-arthritis-registry/