Session Information
Date: Sunday, November 17, 2024
Title: RA – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Biologic and targeted synthetic disease-modifying drugs (b/tsDMARDs) have changed the way rheumatoid arthritis (RA) is managed in recent years. Still, some patients remain symptomatic despite changes in treatment, which has led to the term “difficult-to-treat RA” (D2T AR), a definition proposed by the European Alliance of Associations for Rheumatology (EULAR) in 2020. To date, few studies have been published evaluating the drug survival and safety of b/tsDMARDs in patients with D2T RA. The aim of this study is to compare safety and retention rate of b/tsDMARDs in those patients with a diagnosis of D2T RA using real-world data from a nationwide registry of rheumatic diseases.
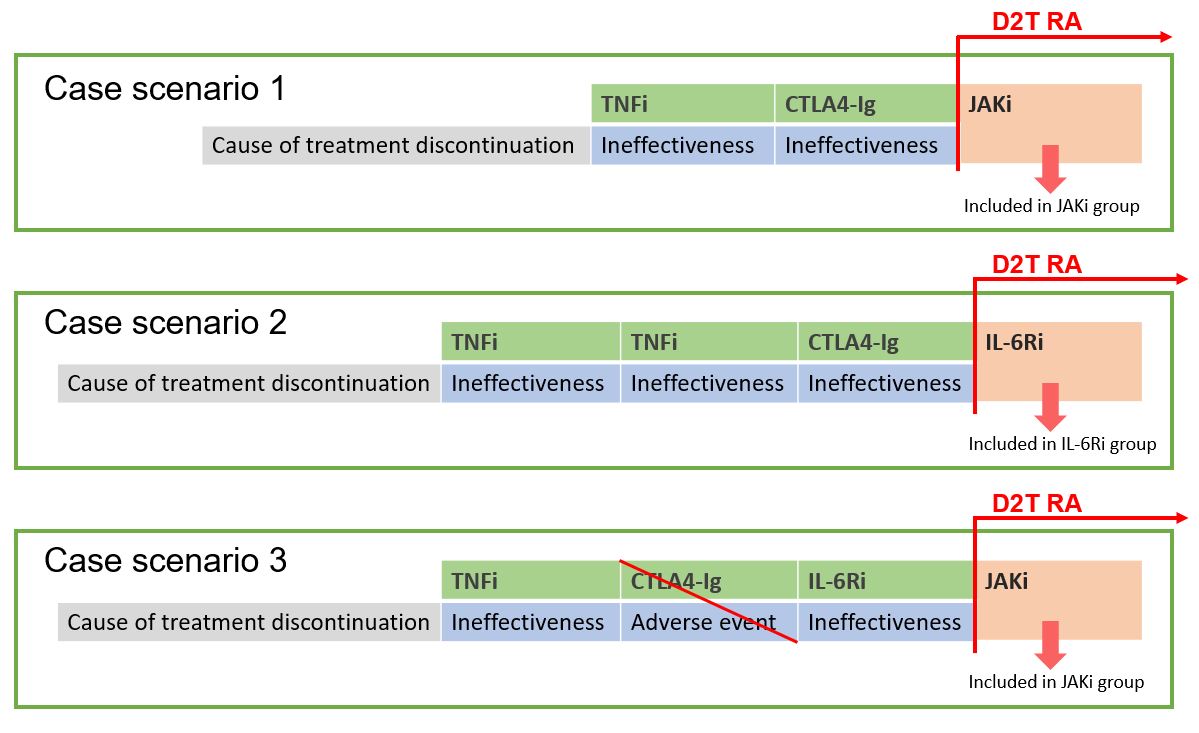
Methods: Longitudinal, observational, and retrospective study in adult patients with a diagnosis of D2T RA included in a national safety registry of rheumatic patients who have received treatment with b/tsDMARDs. The EULAR definition of D2T AR was adapted according to the information available in the registry: patients who have received at least 2 b/tsDMARD with different mechanisms of action, that were discontinued due to ineffectiveness (Figure 1). Data on clinical and demographic characteristics, comorbidities, treatment lines and duration, disease activity indices, and adverse events (AEs) were also collected. The retention rate was analyzed using Kaplan-Meier curves considering the moment in which the patient meets D2T AR criteria as baseline. Regarding safety assessment, the incidence rate of adverse events was estimated. Poisson regression models were also used, adjusting for different clinical and demographic variables.
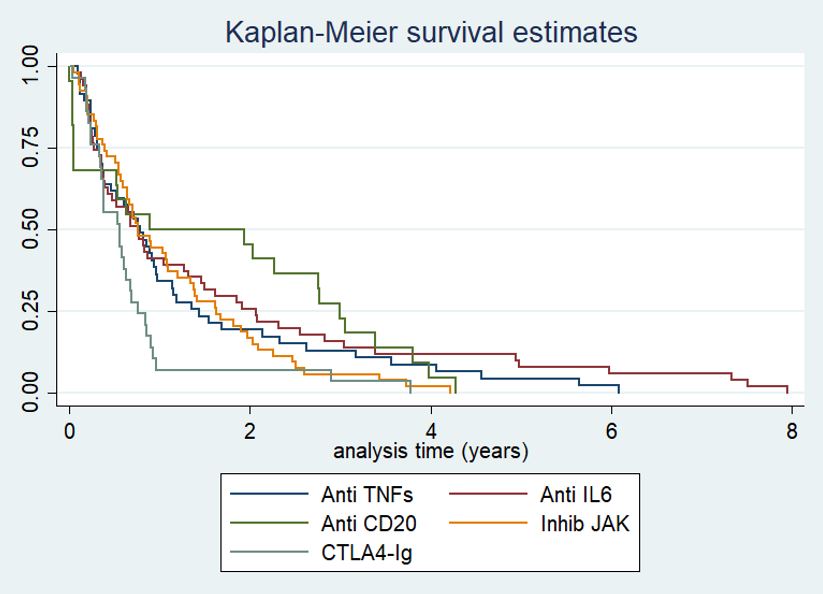
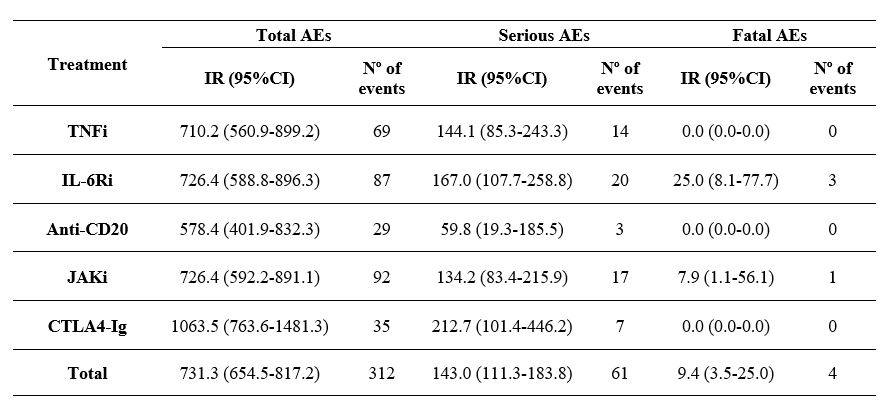
Results: A total of 279 patients with a diagnosis of RA who met the adapted definition of D2T RA were included. Among the incorporated patients: 67, 66, 22, 89 and 35 were classified in the TNF inhibitor (TNFi), interleukin-6 receptor inhibitor (IL-6Ri), anti-CD20 monoclonal antibody (anti-CD20), Janus-kinase inhibitor (JAKi) and cytotoxic T-lymphocyte–associated antigen-4 immunoglobulin (CTLA4-Ig) group, respectively. Concomitant treatment with conventional DMARDs (cDMARDs) was more common in the anti-CD20 group; with methotrexate (MTX) being the most frequently used. No differences were found in the Charlson Comorbidity Index between the groups. Regarding drug retention, no statistically significant differences were detected except for the CTLA4-Ig group, which presented lower persistence (Figure 2). In terms of safety, 312 AEs were recorded: 61 serious AEs (IR 143.0; 95% CI: 111.3-183.8) and 4 fatal AEs (IR 9.4; 95% CI: 3.5-25.0) (Table 1). The most frequent AEs were infections (n: 98; 31.4%), followed by injuries, poisonings and procedural complications (n: 23; 7.4%). Patients in the CTLA4-Ig group showed the highest risk of AEs (IR 1063.5; 95% CI: 763.6-1481.3) and serious AEs (IR 212.7; 95% CI: 101.4-446.2). However, fatal AEs occurred in the IL-6Ri (n: 3) and JAKi (n: 1) group.
Conclusion: CTLA4-Ig presented lower drug retention rate and a higher AEs rate compared to the rest of the drugs in this D2T RA population, which could help guide therapeutic options in these patients. Further research is required in this regard to develop better recommendations.
To cite this abstract in AMA style:
Quevedo-Rodríguez A, Otero-Varela L, Sánchez-Alonso F, Pérez-Vera Y, Manero-Ruiz J, Campos-Fernández C, Manrique-Arija S, Vela-Casasempere P, Mera-Varela A, Díaz C, Movasat A, Garcia-Magallon B, Ros-Vilamajó I, Perez-Garcia C, Castrejon I. Evaluation of the Survival and Safety of Biologic and Targeted Synthetic DMARD in Patients with Difficult-to-Treat RA Using Real-World Data from a Nationwide Registry of Rheumatic Diseases [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/evaluation-of-the-survival-and-safety-of-biologic-and-targeted-synthetic-dmard-in-patients-with-difficult-to-treat-ra-using-real-world-data-from-a-nationwide-registry-of-rheumatic-diseases/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-the-survival-and-safety-of-biologic-and-targeted-synthetic-dmard-in-patients-with-difficult-to-treat-ra-using-real-world-data-from-a-nationwide-registry-of-rheumatic-diseases/