Session Information
Date: Sunday, November 7, 2021
Title: SLE – Diagnosis, Manifestations, & Outcomes Poster II: Manifestations (0855–0896)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Cognitive dysfunction (CD) affects approximately 40% of SLE patients (1), impacting on employment, daily function, and quality of life (2)(3). Diagnostic neuropsychological testing is time-consuming and requires specialised staff, limiting access and resulting in under-detection in routine practice. Therefore, effective screening of SLE patients in the clinical setting is essential in selecting patients for formal cognitive testing. The Montreal Cognitive Assessment (MoCA) is a brief screening tool, initially designed to screen for mild cognitive impairment (MCI) or dementia, which preliminary studies suggest may have utility in SLE. This study aimed to comprehensively evaluate the MoCA as a screening tool for CD in SLE.
Methods: We tested SLE patients (n=95) and demographically matched healthy control (HC) participants (n=48) using the MoCA and the one-hour neuropsychiatric test battery recommended by the ACR for use in SLE (4). CD in SLE patients was defined using standard deviations from the HC group mean. We compared three CD definitions, all based on thresholds from the 2007 ACR response criteria for neurocognitive impairment in SLE clinical trials (5). We used regression analyses to determine associations between MoCA and cognitive test scores, and assessed sensitivity, specificity, positive predictive value and negative predictive value of various MoCA cut-offs for predicting CD. We then determined the diagnostic accuracy of MoCA cut-off thresholds using receiver operator curves.
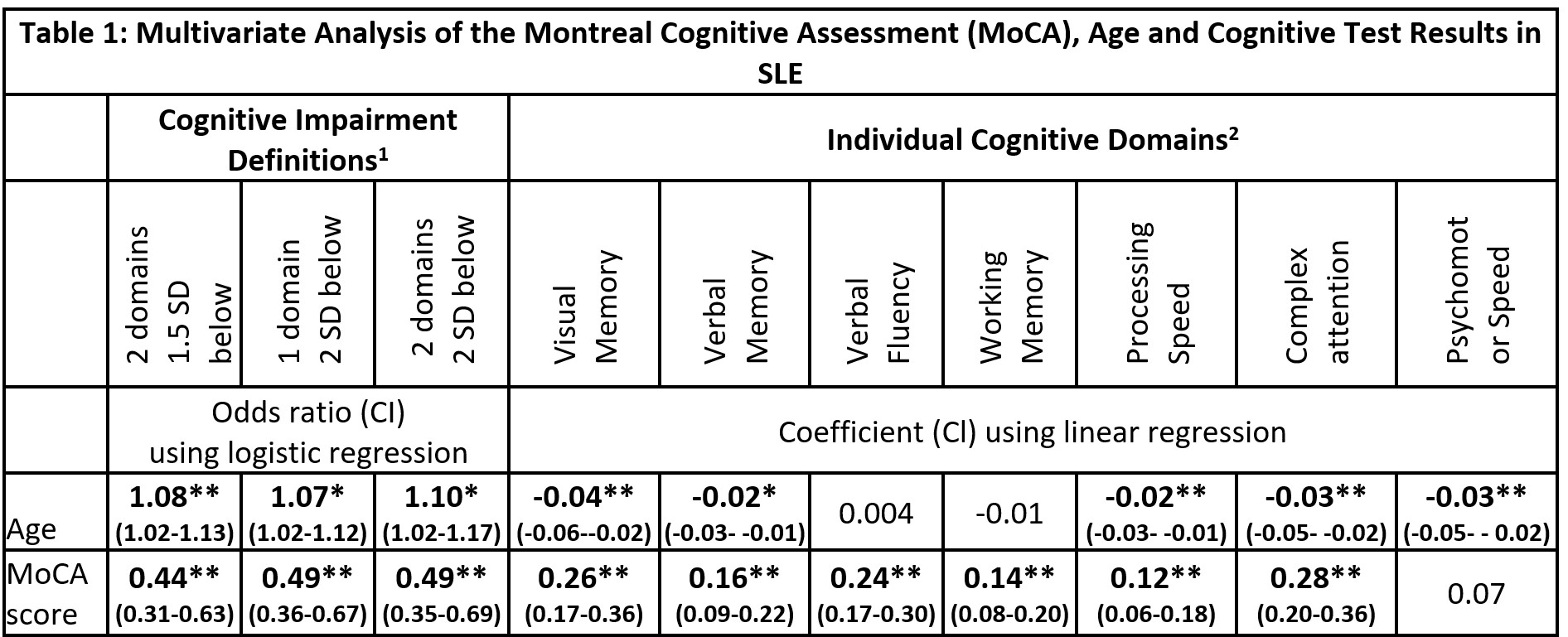
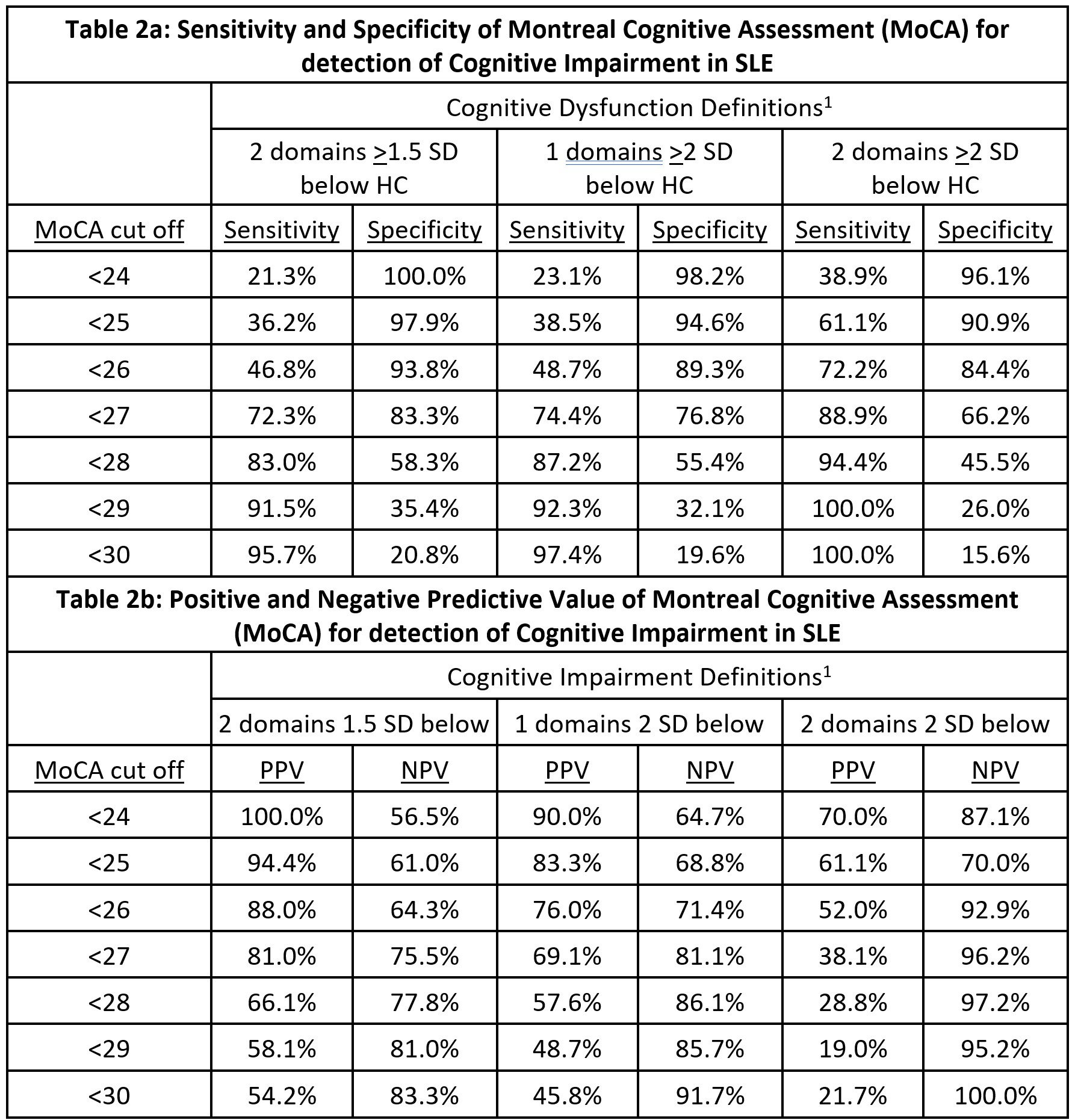
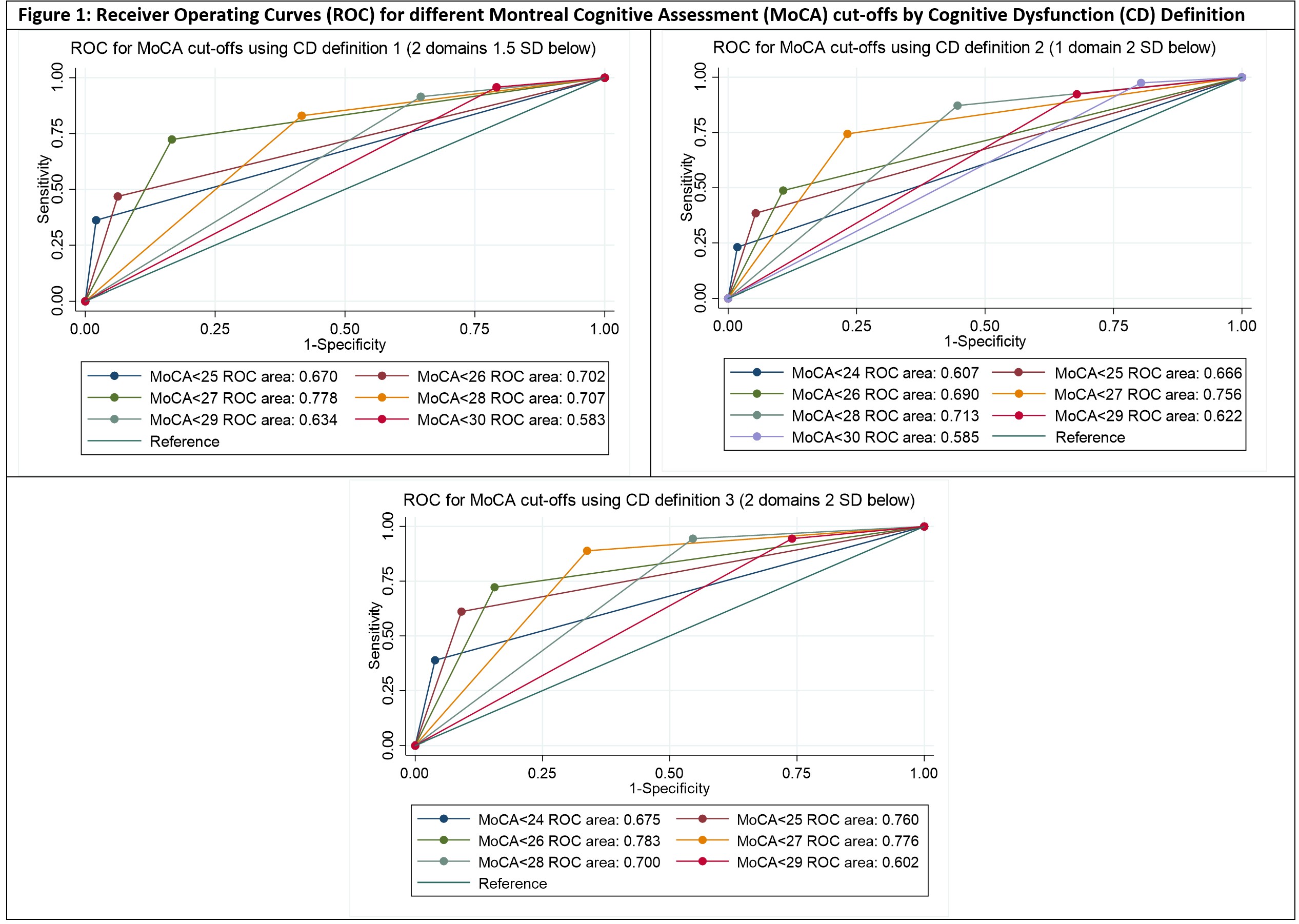
Results: The SLE and HC groups were well matched with no significant differences in age, gender, ethnicity, premorbid IQ or education level. The median age was 45 (range 22-64), 60% were Caucasian and the rest predominantly Asian, and all had good English proficiency. The prevalence of CD in the SLE group varied from 19% to 49% depending on the definition used; the median MoCA score was 26 (range 19-30). On multivariate analysis, MoCA score correlated significantly with nine of the ten cognitive endpoints studied (all p < 0.001; Table 1). Receiver operator curve analysis suggested that the MoCA cut-off of < 27 yielded the highest diagnostic accuracy across the three cognitive impairment definitions (AUC 0.76-0.78; Figure 1). The MoCA cut-off of < 28 had sensitivity of 83-94% with specificity of 46-58%, depending on the impairment definition used (Table 2).
Conclusion: The MoCA correlates strongly with gold-standard cognitive test results in SLE and is sufficiently sensitive for use in screening. We recommend the MoCA cut-off of < 28 as the threshold with the optimal sensitivity for screening for CD in SLE to ensure potential cases are detected. This threshold is higher than the MoCA cut-off used for MCI and dementia. This brief, freely available and practical tool for screening SLE patients for CD should be considered for inclusion in SLE management guidelines.
References: 1) Al Rayes et al. Semin Arthritis Rheum 2018. 2) Arntsen et al. Joint report by Lupus Research Alliance, Lupus Foundation of America & Lupus and Allied Diseases Association 2018. 3) Appenzeller et al. Arthritis Rheum 2009. 4) Liang et al. Arthritis Rheum 1999. 5) Mikdashi et al. Lupus 2007.
1) Impairment defined by number of cognitive domains either 1.5 or 2 SD below healthy control group mean. 2) Specific cognitive tests used for each domain are as follows: Visual Memory – Rey Ostrrieth Complex Figure Test Recall Score, Verbal Memory -California Verbal Learning Test trials 1-5, Verbal Fluency – Controlled Oral Word Association Test FAS Sum, Working Memory – Letter Number Sequencing score, Processing Speed – Coding score, Complex Attention – Trail making test B time inverse, Psychomotor speed – finger tap test dominant hand score. Test scores were expressed as Z-scores in comparison to healthy control group data.
1) Defined by number of cognitive domains either 1.5 or 2 SD below healthy control group mean.
Acronyms: HC – Healthy Control, PPV – Positive Predictive Value, NPV – Negative Predictive Value.
Area under the curve interpretation: 0.7-0.9 = moderate accuracy, 0.5-0.7 = low accuracy, ≤0.5 = equal to chance.
To cite this abstract in AMA style:
Raghunath S, Glikmann-Johnston Y, Morand E, Stout J, Hoi A. Evaluation of the Montreal Cognitive Assessment as a Screening Tool for Cognitive Dysfunction in Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/evaluation-of-the-montreal-cognitive-assessment-as-a-screening-tool-for-cognitive-dysfunction-in-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-the-montreal-cognitive-assessment-as-a-screening-tool-for-cognitive-dysfunction-in-systemic-lupus-erythematosus/