Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: The aim is to examine the efficacy and safety of baricitinib (BARI) in Japanese rheumatoid arthritis (RA) patients in real clinical practice and is to perform a subgroup analysis dividing cases into difficult-to-treat RA (D2TRA) and non-D2TRA.
Methods: This study involved 192 patients with RA (39 men, 153 women) who were administered with BARI. We examined patient background, treatment response at 52 weeks after initiation, continuation rate, and adverse events. We analyzed cases categorized as meeting the definition of D2TRA and those that did not in a similar manner.
Results: The mean age of enrolled patients was 66.0±13 years, and the mean disease duration was 13.4±11.4 years. Rheumatoid factor and anti-CCP antibody were positive in 77% and 76% of patients, respectively, with 9.9% of patients being negative for both antibodies. Prednisolone (PSL) and methotrexate (MTX) were co-administered in 74 cases (5.5±3.3 mg/day) and 85 cases (7.9±2.7 mg/week), respectively. There were 56 naive cases of biological agents/JAK inhibitors, 58 cases who received a second agent, and 78 cases who were introduced to agents after the third. The continuation rate at 52 weeks after starting BARI was 76%, and CDAI and DAS28-ESR(4) significantly decreased from 17.3±11.2, 4.0±1.5 to 5.2±4.5 (p< 0.05), 2.6±0.9 (p< 0.05), respectively. The number of PSL co-administered cases at 52 weeks decreased to 38 cases (3.7±2.1 mg). The occurrence of herpes zoster during the observation period was 4 cases (2.1%). Events leading to the discontinuation of BARI included 32 cases of primary ineffectiveness, 3 cases of secondary ineffectiveness, 10 cases of adverse events (disseminated herpes zoster, pericarditis, dizziness, onset of dermatomyositis, diverticulitis, death due to pneumonia, urticaria, anemia, ileus, mood discomfort), and 1 unknown case. There were 62 cases of D2TRA and 130 of non-D2TRA. No significant differences were found in the ratio of females, age at initiation, disease duration of RA, proportion of the initial BARI dose of 4 mg/day, positive rate or titer of anti-CCP antibodies. While there was no difference in the positive rate of rheumatoid factor, the RF levels were significantly higher in D2TRA (262 vs 138 IU/l, p< 0.05). The continuation rate at 52 weeks after starting BARI was 62% for D2TRA and 81% for non-D2TRA, but no significant difference was observed. CDAI significantly decreased from initiation to 52 weeks, with no significant difference between the two groups. The DAS28-ESR(4) before initiation was 4.27 for D2TRA and 4.00 for non-D2TRA, showing no significant difference, but it was significantly higher for D2TRA at 52 weeks after initiation (2.94 vs 2.47, p< 0.05). The DAS remission rate was 40% for D2TRA and 65% for non-D2TRA. The ineffectiveness discontinuation cases were 26% for D2TRA and 15% for non-D2TRA. The discontinuation cases due to adverse events were 8% for D2TRA and 4.6% for non-D2TRA, with no significant differences observed.
Conclusion: BARI significantly reduced disease activity and showed a high continuation rate. Serious adverse events were infrequent, confirming efficacy and safety in real clinical practice. BARI did not demonstrate the comparable efficacy in D2TRA cases compared to non-D2TRA.
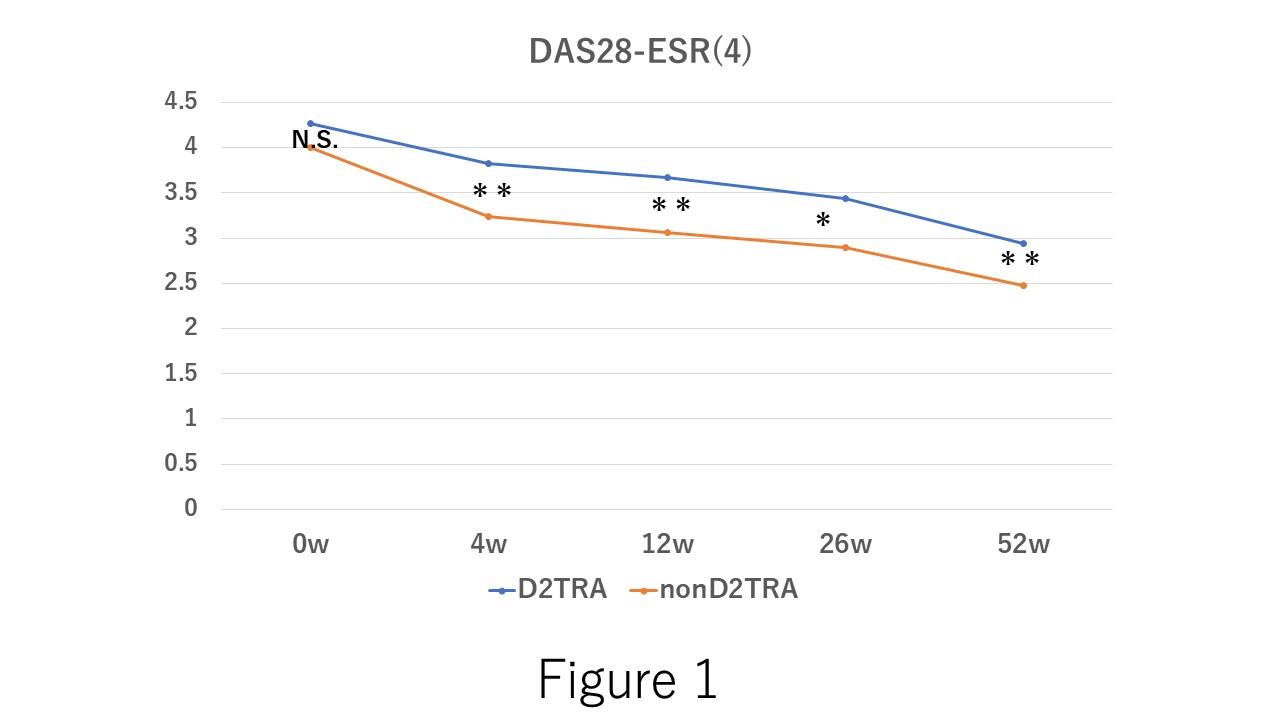 Table1. The demographic data of difficult-to-treat RA (D2TRA) and non D2TRA groups. Rheumatoid factor value at the baseline in D2TRA shows significantly higher than that in nonD2TRA.
Table1. The demographic data of difficult-to-treat RA (D2TRA) and non D2TRA groups. Rheumatoid factor value at the baseline in D2TRA shows significantly higher than that in nonD2TRA.
.jpg) Figure 1. The time course of DAS28-ESR(4) after the baricitinib. In the administration of baricitinib, no significant difference between the difficult-to- treat RA (D2TRA) and non D2TRA. However, 4, 12, 26, and 52 weeks after the administration, DAS28-ESR(4) in D2TRA is significantly higher than that in nonD2TRA. N.S.; not significant difference, *; p < 0.05, **; p < 0.01.
Figure 1. The time course of DAS28-ESR(4) after the baricitinib. In the administration of baricitinib, no significant difference between the difficult-to- treat RA (D2TRA) and non D2TRA. However, 4, 12, 26, and 52 weeks after the administration, DAS28-ESR(4) in D2TRA is significantly higher than that in nonD2TRA. N.S.; not significant difference, *; p < 0.05, **; p < 0.01.
To cite this abstract in AMA style:
Kondo N, Hao N. Evaluation of the Clinical Outcome of Baricitinib in Patients with Rheumatoid Arthritis from Multi-center Collaborative Study in Japan: Sub-analysis for Difficult-to-treat RA. [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/evaluation-of-the-clinical-outcome-of-baricitinib-in-patients-with-rheumatoid-arthritis-from-multi-center-collaborative-study-in-japan-sub-analysis-for-difficult-to-treat-ra/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-the-clinical-outcome-of-baricitinib-in-patients-with-rheumatoid-arthritis-from-multi-center-collaborative-study-in-japan-sub-analysis-for-difficult-to-treat-ra/
