Session Information
Title: Rheumatoid Arthritis - Clinical Aspects: Novel Biomarkers and Other Measurements of Disease Activity
Session Type: Abstract Submissions (ACR)
Background/Purpose: Based on treat-to-target guidelines, the goal of treatment should be remission. Definitions for remission recommended by the ACR/EULAR task force include joint counts and a laboratory test and were selected to predict later good radiographic and functional outcomes.1 Despite these guidelines, rheumatologists still do not regularly measure disease activity. RAPID3, developed as a tool for monitoring disease activity, is advantageous because it requires only patient input. This analysis evaluated the performance of RAPID3 with minimal joint count (for physician input), compared with ACR/EULAR proposed remission definitions, to predict future good radiographic + functional outcome.
Methods: LITHE was a 5-year, double-blind, phase 3 study of tocilizumab (TCZ) in patients with moderate to severe RA who had inadequate responses to MTX therapy.2 Patients were randomly assigned 1:1:1 to receive placebo + MTX, TCZ 4 mg/kg + MTX, or TCZ 8 mg/kg + MTX for 24 weeks, with an option to escape starting at week 16. Associations between various year 1 measures were evaluated against rates of good radiographic + functional outcome at year 2, specifically no worsening of Genant-Sharp Score (GSS) and HAQ-DI, and HAQ-DI ≤0.5.1 Logistic regression was used to estimate odds ratio (OR), positive predictive value (PPV), negative predictive value (NPV), and sensitivity/specificity of the measures.
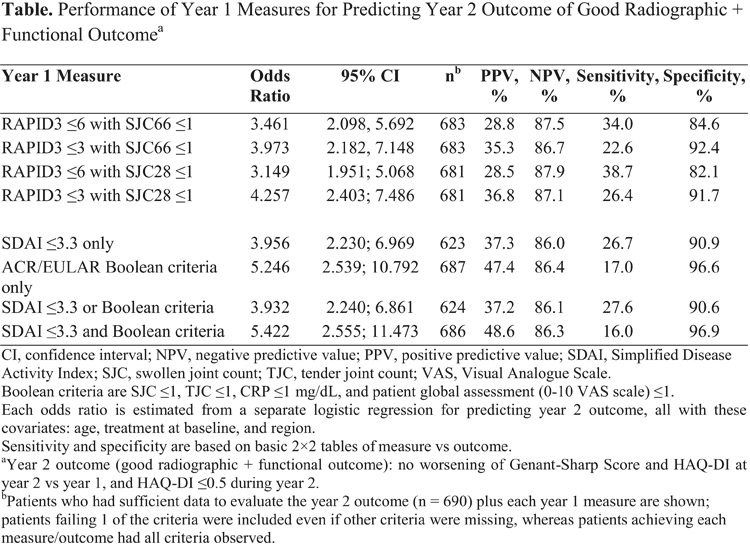
Results: In total, 690 patients had sufficient 2-year data to evaluate year 2 outcome: 73% of patients had no worsening of GSS score, 58% had no worsening of HAQ-DI, 22% had HAQ-DI ≤0.5, and 15% met all 3 criteria. ORs with 95% confidence intervals (CIs) for all year 1 measures were >1, indicating that all were statistically significantly associated with year 2 outcome, though no one measure had superior association (p < 0.05 for all; overlapping 95% CIs for OR). NPV and specificity were high (>80%), but PPV and sensitivity for all measures were low (range, 28-49% and 16-39%, respectively), which meant the measures could successfully identify patients who did not achieve the year 2 outcome but were not very good at identifying patients who achieved the outcome (Table).
Conclusion: Consistent with publications on other therapies/populations,3-5 these results suggest the RAPID3 plus ≤1 joint count performed similarly to more complex measures. However, given that no single measure had especially high PPV or sensitivity, it is important that the patient be fully evaluated to understand what individual factors might contribute to his or her long-term prognosis.
References: 1. Arthritis Rheum 2011;63:573. 2. J Rheumatol 2013;40:113. 3. Arthritis Care Res 2011;63:1142. 4. Autoimmune Dis 2013:367190. doi: 10.1155/2013/367190. 5. J Rheumatol 2011;38:2565.
Disclosure:
M. J. Bergman,
Pfizer Inc, Johnson and Johnson,
1,
AbbVie,
2,
AbbVie, Johnson and Johnson, Pfizer, Amgen, Celgene, Genentech,
5,
AbbVie, UCB, Celgene,
8;
J. Yourish,
Genentech/Roche,
3;
J. Pei,
Genentech/Roche,
3;
J. Devenport,
Genentech/Roche,
3;
W. Reiss,
Genentech/Roche,
3;
E. Keystone,
Abbott, Amgen, AstraZeneca, BMS, F. Hoffmann-La Roche, Janssen, Lilly, Novartis, Pfizer Sanofi-Aventis, UCB,
2,
Abbott Laboratories, AstraZeneca, Biotest, BMS, F. Hoffmann-La Roche, Genentech, Janssen, Lilly, Merck, Pfizer, UCB,
5,
Abbott, AstraZeneca, BMS Canada, F. Hoffmann-La Roche, Janssen, Pfizer, UCB, Amgen,
8.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-rapid3-with-minimal-joint-count-and-acreular-provisional-remission-definitions-as-predictors-of-future-good-radiographic-functional-outcome-in-a-double-blind-phase-3-randomized-cont/