Session Information
Date: Sunday, November 13, 2022
Title: Osteoporosis and Metabolic Bone Disease – Basic and Clinical Science Poster
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: The most commonly used drugs for glucocorticoid-induced osteoporosis (GIOP) is bisphosphonates, which include oral alendronate and risedronate, and intravenous zoledronic acid. Among them, zoledronic acid is a convenient treatment option that allows compliance to be advantageous over other bisphosphonates. In this study, we aim to evaluate preference and patient satisfaction as well as the efficacy of zoledronic acid compared to other bisphosphonates.
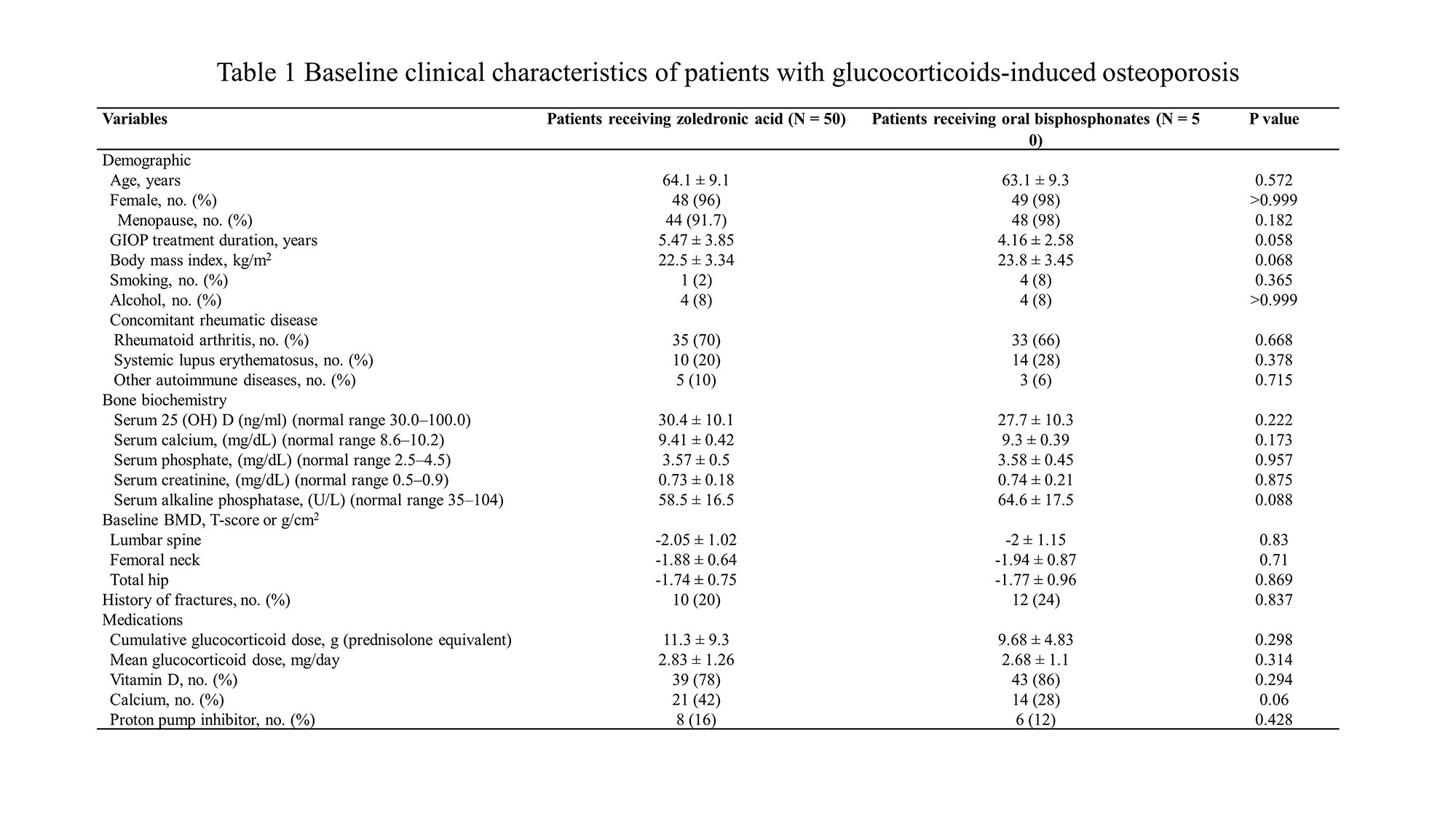
Methods: Fifty patients with continued osteoporosis or new fractures were recruited in follow-up bone densitometry after taking oral bisphosphonates for at least 1 year in patients diagnosed with GIOP during treatment for autoimmune diseases. After 1 year of treatment with zoledronic acid, patients completed the survey to rate their preference and satisfaction. The treatment efficacy was analyzed by comparing the changes in bone density and fractures with patients maintaining oral bisphosphonates as controls.
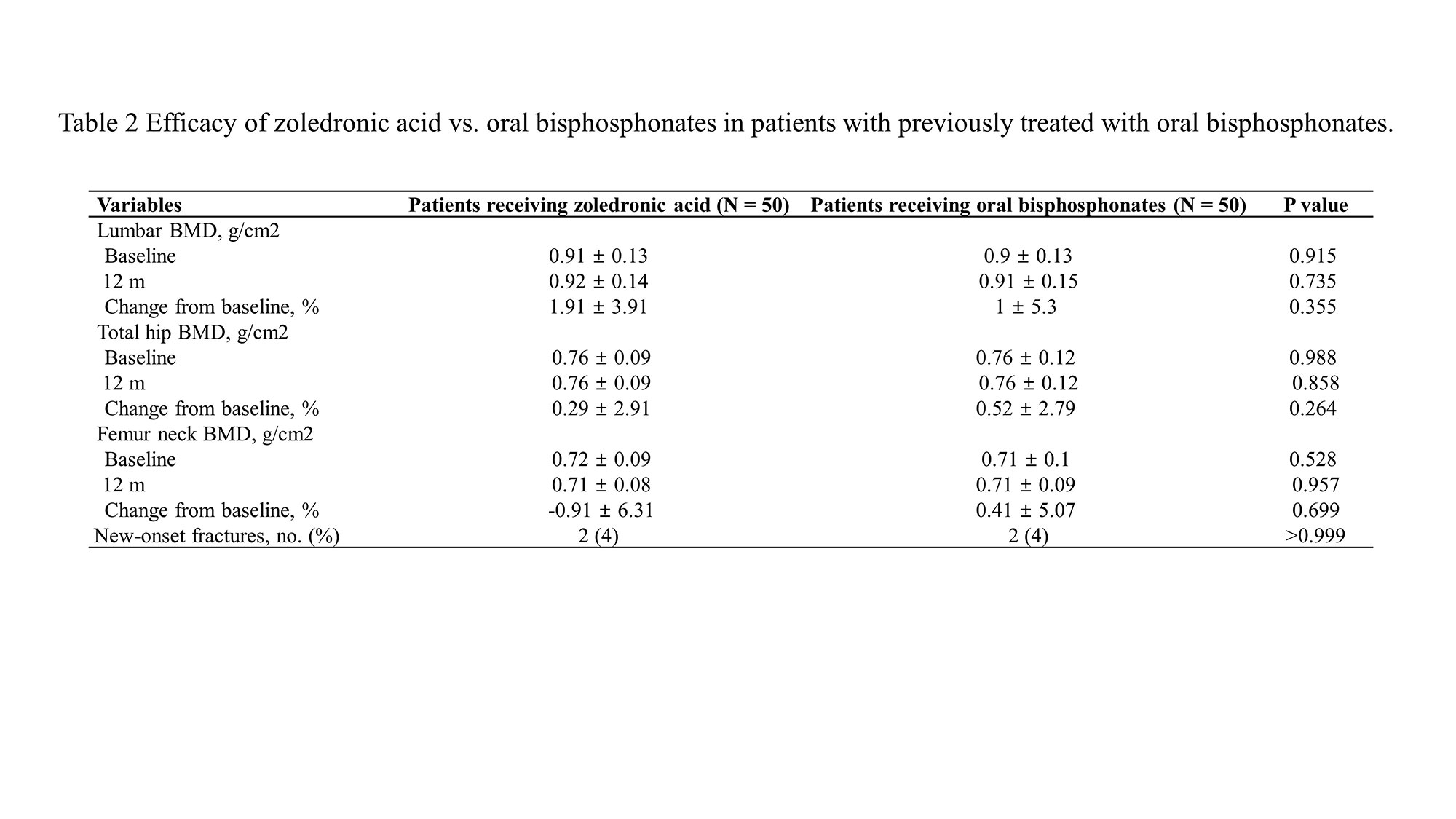
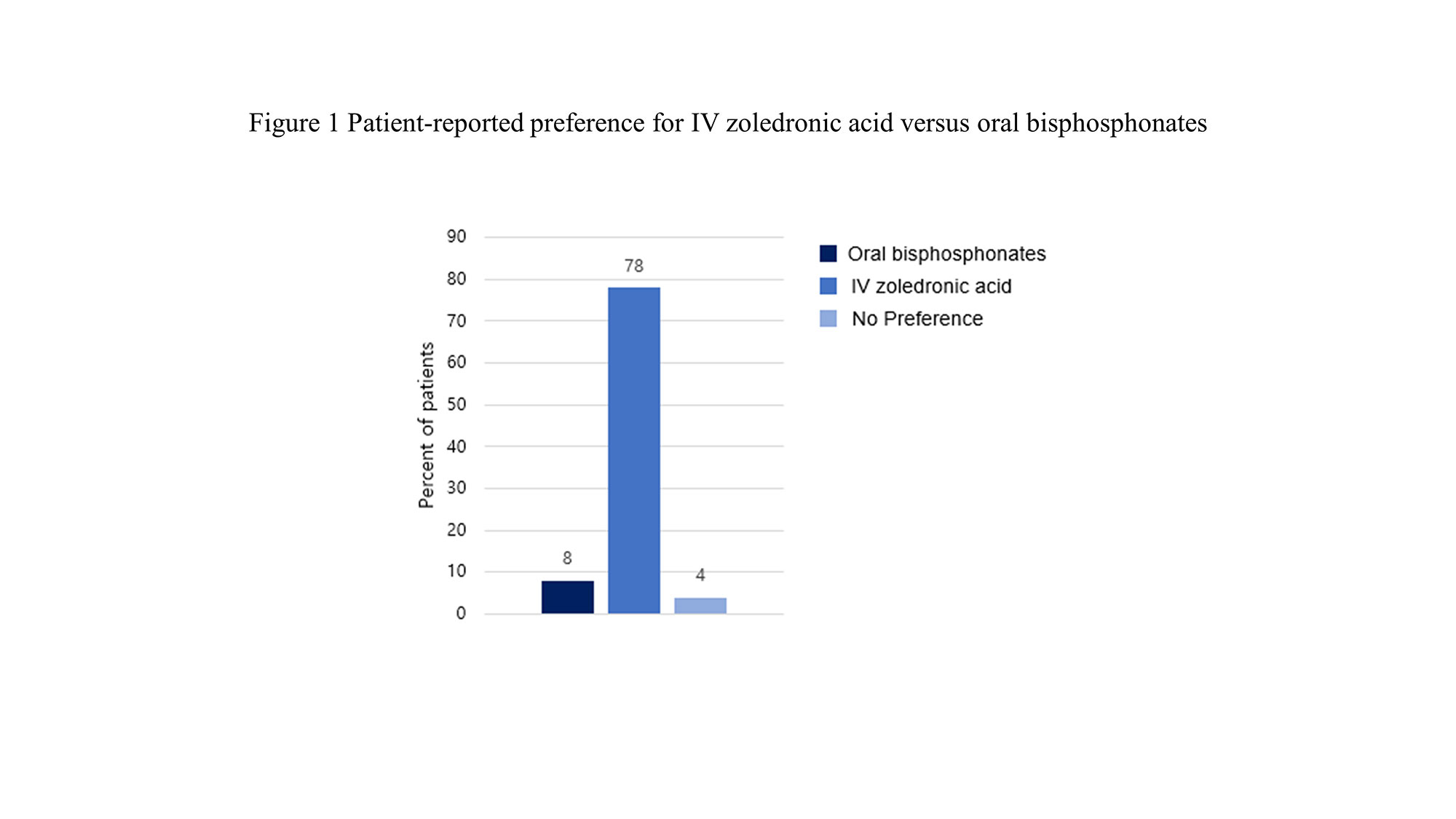
Results: The mean age of patients was 64.1 years, 96% were female, and the mean duration of GIOP was 5.5 years. There was no difference in the cumulative glucocorticoid doses in zoledronic acid group and oral bisphosphonate group. Thirty-nine patients (78%) preferred and were more satisfied with intravenous zoledronic acid over oral bisphosphonates, and satisfaction was greatly affected by the administration interval and convenient regimen. The infusion-related adverse events of zoledronic acid were only 2 patients (4%). In terms of efficacy, there were no significant differences in annualized percentage change in bone density in the lumbar spine (1.9 ± 3.91 g/cm2 vs. 1 ± 5.3 g/cm2, p = 0.355), femur neck (-0.91 ± 6.31 g/cm2 vs. 0.41 ± 5.07 g/cm2, p = 0.264), and hip (0.29 ± 2.91 g/cm2 vs. 0.41 ± 5.07 g/cm2, p = 0.888) between patients who received zoledronic acid and those who took oral bisphosphonates. The occurrence of new fractures was two in each of the two groups, showing no difference.
Conclusion: In our study, zoledronic acid was preferred and more satisfactory by patients, and the treatment efficacy for osteoporosis was similar to oral bisphosphonates. Therefore, zoledronic acid is recommended as an appropriate treatment for GIOP in patients with autoimmune disease.
To cite this abstract in AMA style:
Kim J, Jung J, Kim H, Suh C. Evaluation of Preference and Efficacy in the Treatment of Glucocorticoid-induced Osteoporosis in Patients with Autoimmune Diseases Receiving Zoledronic Acid [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/evaluation-of-preference-and-efficacy-in-the-treatment-of-glucocorticoid-induced-osteoporosis-in-patients-with-autoimmune-diseases-receiving-zoledronic-acid/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-preference-and-efficacy-in-the-treatment-of-glucocorticoid-induced-osteoporosis-in-patients-with-autoimmune-diseases-receiving-zoledronic-acid/