Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Inhibition of the Janus kinase-signal transducers and activators of transcription (JAK-STAT) pathway have demonstrated efficacy in immune-mediated diseases and haves been identified as therapeutic targets for the treatment of rheumatoid arthritis (RA). Differences in JAK inhibitor specificity for JAK1, JAK2, JAK3, and TYK2 may influence their safety profiles, but the mechanism is not known. Selective JAK1 inhibition by filgotinib (FIL) may improve the risk-benefit profile by minimizing non-JAK1–related adverse events. JAK2 inhibition is associated with cytopenias, while JAK3 inhibition has been associated with increased risk for opportunistic infections and chronic low-grade inflammation. In clinical trials, FIL did not negatively impact hemoglobin, LDL/HDL ratios, or natural killer (NK) cell counts.1-3 Here we compare the in vitro profile of JAK inhibitors with different JAK selectivity profiles at clinically relevant doses to calculate the overall kinetics of suppression of erythroid progenitor cell expansion and maturation, NK cell proliferation, and liver X receptor (LXR) agonist-induced cholesteryl ester transfer protein (CETP) expression.
Methods: JAK inhibitors (FIL, FIL metabolite [GS-829845], baricitinib [BARI], tofacitinib [TOFA], and upadacitinib [UPA]) were evaluated in vitro in human cell-based assays: growth of erythroid progenitors from human cord blood CD34+ cells using a HemaTox™ liquid expansion assay, IL-15–induced NK cell proliferation, and LXR agonist-induced CETP expression in the hepatic cell line (HepG2). Using IC50s generated from these assays and the reported human plasma concentrations of the JAK inhibitors from clinical studies,4-6 we calculated the target coverage for each compound at clinically relevant doses. The activity of FIL in humans was based on a PK-PD modeling algorithm7 of FIL + GS-829845.
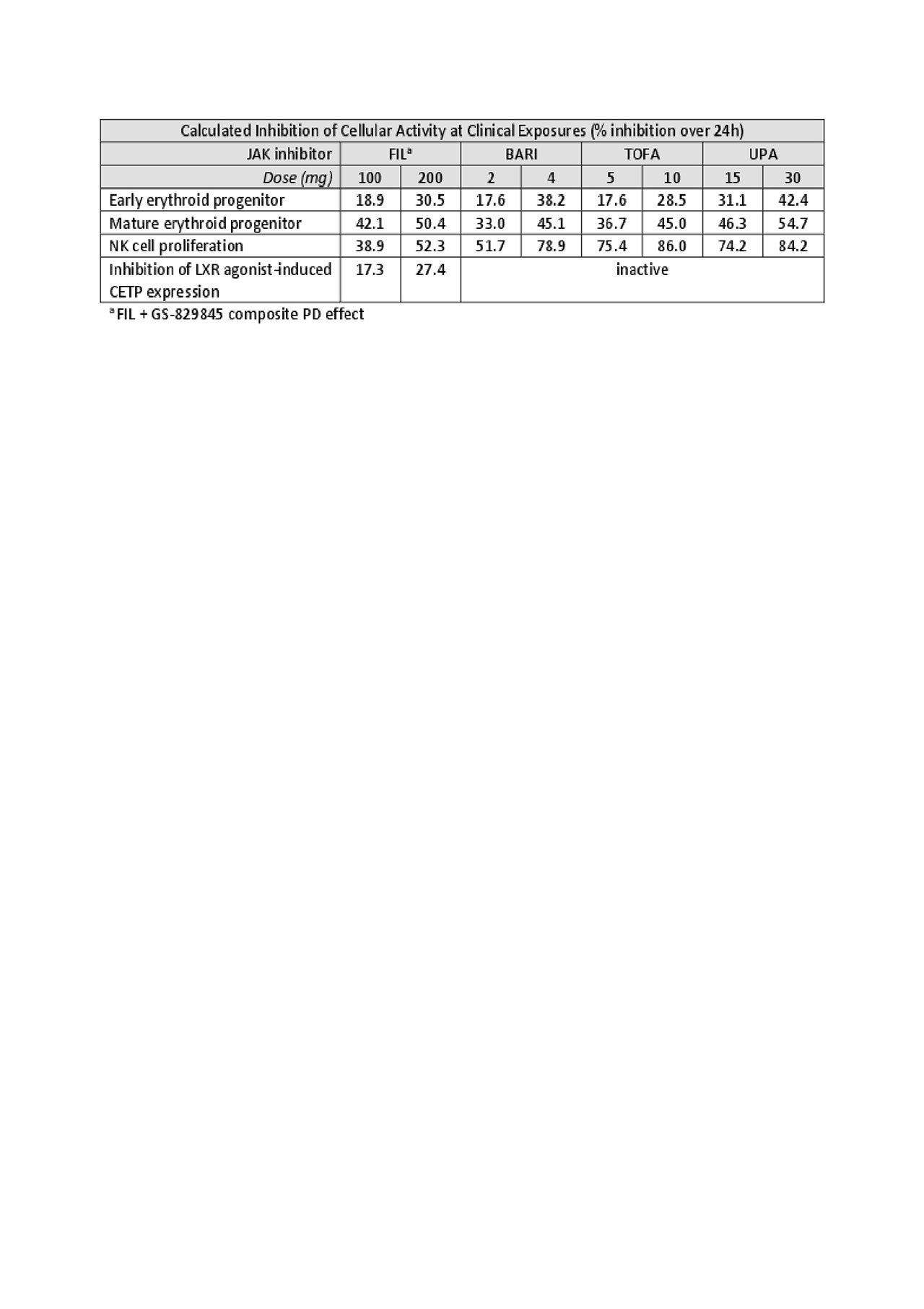
Results: In vitro dose-response assay results were obtained for each inhibitor. Based on these results, human exposure data, and modeled PK-PD relationships, FIL 100 mg and FIL 200 mg result in lower calculated cellular inhibition than the other JAK inhibitors at clinical exposures for NK cell proliferation and CETP expression. There was no obvious difference in the effect of FIL on erythroid progenitor cell differentiation or maturation versus the other JAK inhibitors in these experiments.
Conclusion: JAK1 selectivity of FIL resulted in less inhibition of NK cell proliferation compared with BARI, TOFA, and UPA. FIL also reduced LXR agonist-induced CETP expression, while the other inhibitors did not alter these levels. These results provide a potential mechanistic link to the observed reduction of CETP concentration and activity following FIL treatment, and the observed reduction in LDL:HDL in RA patients.8
References:
- Kavanaugh A, et al. Ann Rheum Dis. 2017;76:1009-1019.
- Westhovens R, et al. Ann Rheum Dis. 2017;76:998-1008.
- MC Genovese, et al. ACR 2018, Abstract L06.
- Shi JG, et al. J. Clin Pharmacol. 2014;54:1354-1361.
- Lamba M, et al. J Clin Pharmacol. 2016;56:1362-1371.
- Mohamed MF, et al. Clin Pharmacokinet. 2016;55:1547-1558.
- Tuk B, et al. J Pharmacol Exp Ther. 1999;289:1067-1074.
- Galien R, et al. Arthritis Rheumatol. 2015; 67(suppl 10).
To cite this abstract in AMA style:
Di Paolo J, Downie B, Meng A, Mollova N, Yu Y, Han P. Evaluation of Potential Mechanisms Underlying the Safety Observations of Filgotinib in Clinical Studies in RA [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/evaluation-of-potential-mechanisms-underlying-the-safety-observations-of-filgotinib-in-clinical-studies-in-ra/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-potential-mechanisms-underlying-the-safety-observations-of-filgotinib-in-clinical-studies-in-ra/