Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Abatacept (ABA) has proven efficacy in autoimmune diseases and is being evaluated in pSS. The purpose of this investigation was to evaluate the pharmacokinetics (PK) and immunogenicity (IM) of subcutaneous (SC) ABA in pSS and RA patients.
Methods: One hundred eighty eight subjects diagnosed with active, moderate to severe pSS who meet the 2016 ACR/EULAR Classification Criteria for pSS and have a ESSDAI disease activity score of at least 5 at screening and are refractory to symptomatic or local therapy (e.g., NSAIDs) were randomized 1:1 to 125 mg SC weekly ABA (95 subjects) or placebo (92 subjects) in a double-blind, 24-week, placebo-controlled study (NCT02915159, IM101603) [Ref. 1].
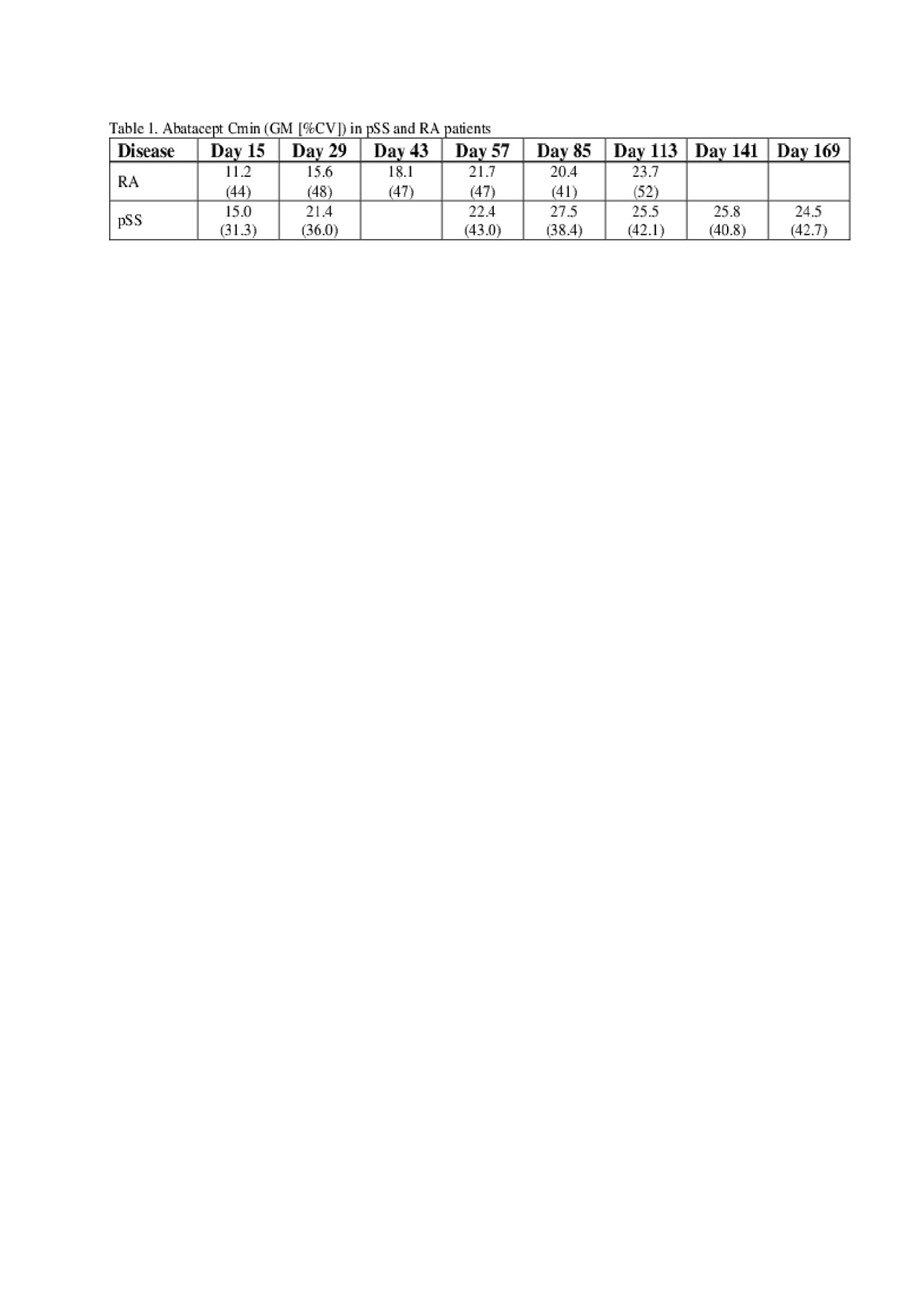
ABA trough (Cmin) samples were collected in all subjects during the treatment window of 365 days and semi-intensive PK samples were collected in a subset of subjects during the first 85 days. Serum ABA concentrations and anti-drug antibodies (ADA) were measured using a validated enzyme immunoassay method and an electrochemiluminescence assay, respectively.
Graphical analyses evaluated the PK profile and Cmin of ABA in pSS and compared to the ACCOMPANY study (IM101173) in RA patients [Ref. 2]. IM101173 was a 4-month open-label Phase 3b study to evaluate the immunogenicity and safety of SC ABA administered 125 mg weekly in the absence of an IV loading dose, when given as monotherapy versus with concomitant MTX, and to assess whether the use of concomitant MTX influenced the development of immunogenicity in RA patients with inadequate response to MTX.
Immunogenicity incidence rates were also summarized in the pSS and RA populations.
Results: Geometric mean (GM) Cmin values in pSS patients of > 15 μg/mL were achieved by Day 15 and remain greater than the threshold of 10 μg/mL that is associated with near maximal efficacy response established in RA patients across all body weights who receive the weekly 125 mg SC dosing (Table 1). ABA GM (%CV) Cmin values at steady state (Day 85) were comparable in pSS (27.5 [38.4%] μg/mL) and RA (20.4 [41%] μg/mL) patients.
During the 168-day blinded period, 1 (1.1%, n=90) subject with pSS had a positive antibody response specific to CTLA4 and possibly Ig relative to baseline while on-treatment. During the 4-month open label period, the overall immunogenicity rate was 4.1% (2/49) in RA subjects.
Conclusion: Subcutaneous administration of ABA 125 mg weekly in pSS achieved consistent target steady state trough concentrations to that observed in RA and shown to be efficacious in RA [Ref 3]. SC abatacept elicited minimal immunogenicity and was associated with no effect on PK or safety in either pSS or RA patients.
References:
1. Baer A, et al. EULAR Congress [Abstract OP0039]. June 2019. Madrid, Spain
2. Murthy B, et. al. EULAR Congress. June 2011. London, UK
3. Li X, et. al. J Clin Pharmacol. Vol 59. 2019
To cite this abstract in AMA style:
Gandhi Y, Shaikh M, Vakkalagadda B, Abelian G, Ray N, Wong R, Murthy B. Evaluation of Pharmacokinetics and Immunogenicity Following Subcutaneous Administration of Abatacept in Primary Sjogren ’s Syndrome (pSS) and RA Patients [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/evaluation-of-pharmacokinetics-and-immunogenicity-following-subcutaneous-administration-of-abatacept-in-primary-sjogren-s-syndrome-pss-and-ra-patients/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-pharmacokinetics-and-immunogenicity-following-subcutaneous-administration-of-abatacept-in-primary-sjogren-s-syndrome-pss-and-ra-patients/