Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: The Opioid Epidemic has been declared a public health emergency since 2017. The use of opioids in the acute crystal induced arthritis (ACIA) population has not been well evaluated. Despite availability of effective gout treatments, opioid use remains prevalent in hospitalized patients [1]. Traditionally, treatment for acute flares includes non-steroidal anti-inflammatories, colchicine, and glucocorticoids. Anakinra is a relatively new immunomodulator that has been shown to be non-inferior to traditional treatments for acute gout flares, suggesting that anakinra is an effective alternative treatment for acute gout flares [2]. We proposed to look at the total amount of opioid use in the inpatient population suffering from acute crystal induced arthritis and also evaluate whether anakinra use may reduce opioid prescribing.
Methods: Retrospective chart review of all adult in-patients who were admitted between 2015 through 2018 with gout or pseudogout flare were evaluated. A total of 204 patients met ACR/EULAR criteria for ACIA. Of those, seventeen had received anakinra for ACIA and thirty-four patients receiving traditional treatment were matched with the anakinra group in a 1:2 ratio. The two groups were matched for age, gender and number of joints affected. Data collection included demographics, home medications, comorbidities, and Visual Analog Pain Scale (VAS-pain). Response to treatment was defined as decrease in VAS-pain by more than 2 points on a 0-10 scale at 24, 48, and 72 hours. The total opioid dose in morphine milliequivalents was reviewed for both groups. Repeated measures ANOVA was used to compare each group at each time-point of pain assessment. Tukey-Kramer multiple comparisons test was used for post-hoc comparisons. Significance was set at p< 0.05.
Results: All the patients in traditional treatment group had significantly more use of opioids for their joint pains compared to the anakinra group (p< 0.05). All patients receiving anakinra had statistically significant improvement in VAS-pain compared to the conventional group (p< 0.001). Within the anakinra group, pain was significantly improved at 24 hours (p< 0.05) and at 48 hours (p< 0.001) compared to baseline. The conventional group had significant improvement at 48 hours compared to baseline (p< 0.05) but was not significantly improved at 24 hours (p >0.05). Between groups pain was not different at baseline or at 24 hours (p >0.05). On average the number of days of hospitalization since treatment initiation was nearly 2 days shorter in the anakinra group compared to conventional. In addition, prescription for opioids at discharge was more likely in the traditional treatment group [OR = 3.34, (CI 0.37-30.70)], but this did not reach statistical significance. No significant adverse events were noted in the anakinra group.
Conclusion: A significant amount of opioid analgesia is employed in treating inpatients with ACIA. Significant pain reduction with anakinra highlights the availability of safe alternative for inpatients with ACIA. Additional investigations are warranted to evaluate the appropriate role of opioids in ACIA population and how to control its utilization.
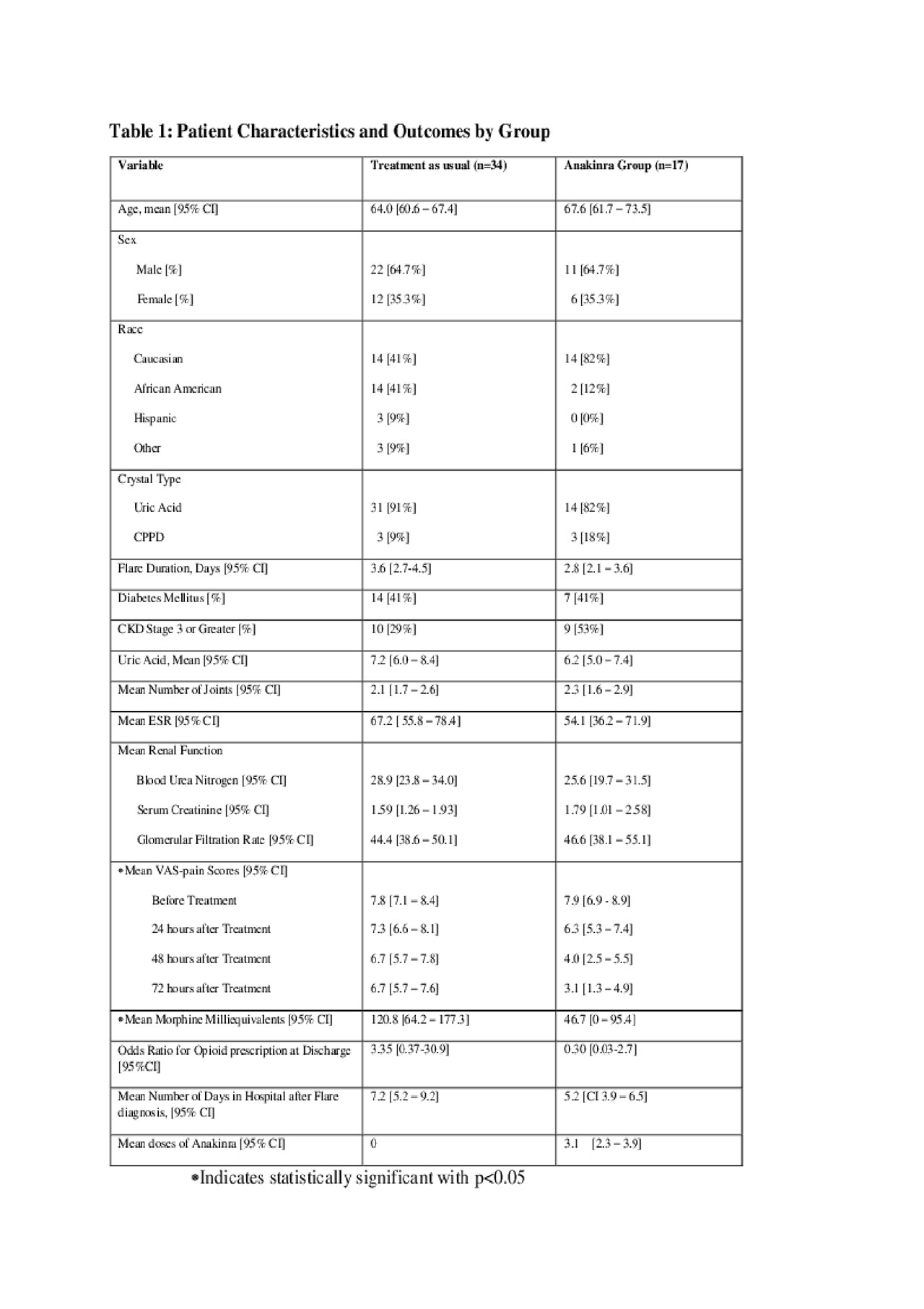
Opioid ACIA Table 1-converted -1-
To cite this abstract in AMA style:
Singh S, Ocon A, Riley M, Tchervenkov J, Peredo-Wende R. Evaluation of Opioid Analgesia in Hospitalized Patients with Acute Crystal Induced Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/evaluation-of-opioid-analgesia-in-hospitalized-patients-with-acute-crystal-induced-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-opioid-analgesia-in-hospitalized-patients-with-acute-crystal-induced-arthritis/
