Session Information
Date: Saturday, November 16, 2024
Title: SpA Including PsA – Diagnosis, Manifestations, & Outcomes Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: The optimal instrument to assess peripheral arthritis and related disease activity in SpA has not yet been identified. In the recent update of the ASAS core set for axSpA, swollen joint count (SJC) was assessed and chosen for the measurement of peripheral manifestations, but no other instruments and no composite scores were considered for the assessment of this domain. The objective of this study was to assess the construct validity, including known-groups discrimination, of the currently available disease activity instruments assessing peripheral arthritis in SpA and to investigate whether there are differences across SpA phenotypes.
Methods: In this analysis from the ASAS-PerSpA study, patients with a diagnosis of axSpA, pSpA or PsA were included.
The disease activity instruments evaluated were patient global assessment (PGA), BASDAI, ASDAS,
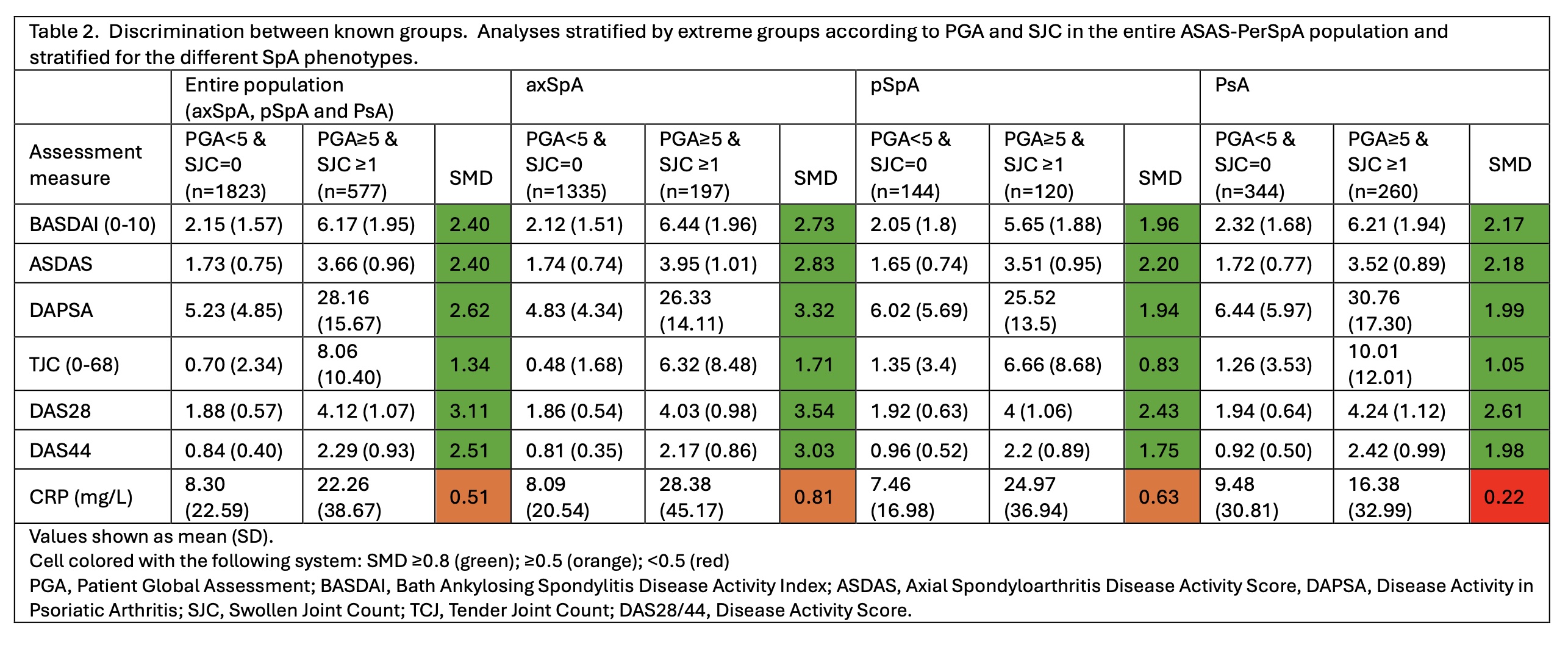
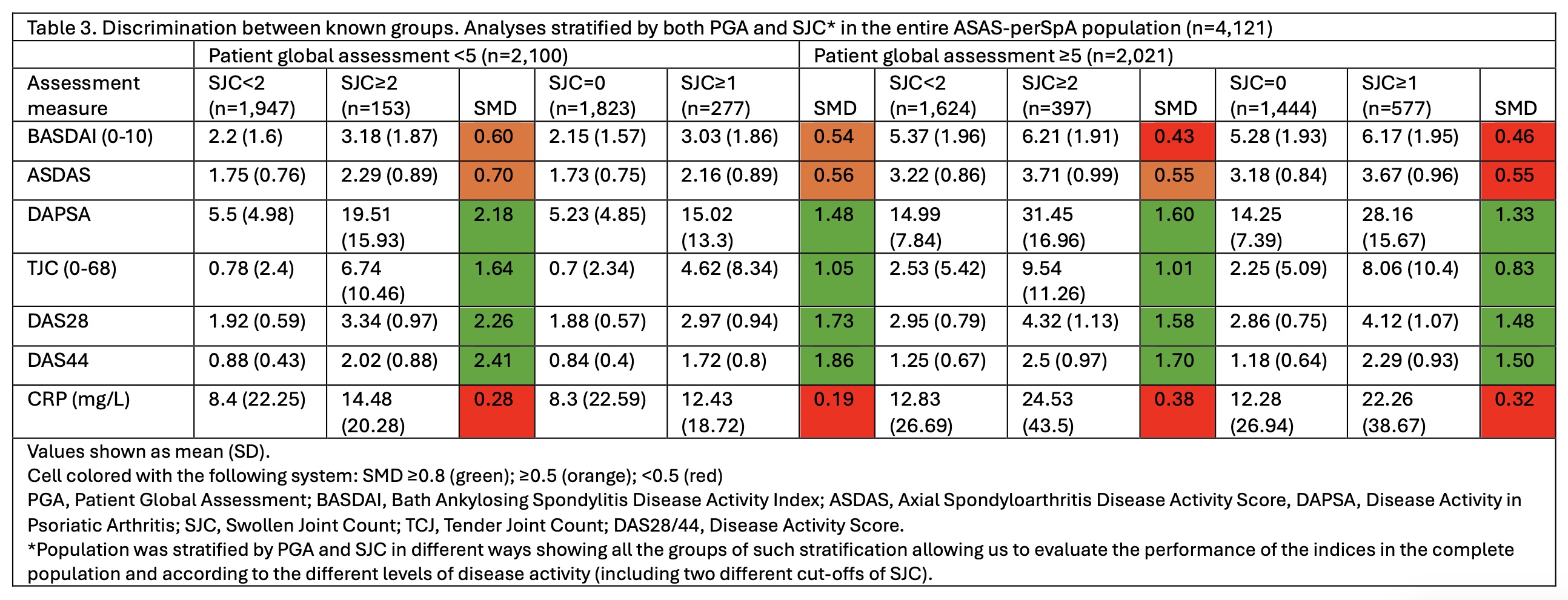
DAPSA, SJC, tender joints count (TJC), DAS28, DAS44, and C-reactive protein (CRP). Construct validity was assessed through correlations with external constructs (BASFI, ASAS-HI and EuroQoL), and known-groups discrimination. Firstly, hypotheses for the strength of the correlation between the assessed instruments and external constructs were established. Then, Spearman correlations were calculated to assess whether the hypothesis for the strength of each correlation (between the assessed instruments and the external constructs) was accepted/rejected. Construct validity was considered as “good”, “moderate” or “poor” if ≥75%, 50-75% or < 50% of the hypotheses were confirmed. For the discrimination between known-groups, patients were stratified into active/inactive groups based on a combination of PGA (≥5/< 5) and SJC (≥1/0 and also ≥2/< 2, median SJC). Standardized mean differences (SMDs) of all individual instruments were compared. An SMD with higher absolute value indicates better discriminatory ability, and an SMD above 0.8 is considered as ‘good’.
Results: In total, 4,121 patients were included (mean age 45 (SD 14) years, 61% males). When assessing the construct validity through correlations, all instruments had an excellent performance (100% of confirmed hypotheses) (Table 1). In the entire population, all disease activity measures, except for CRP, presented SMDs≥0.8 (good performance), with a higher SMD being observed for DAS28 followed by DAPSA, DAS44, BASDAI and ASDAS (Table 2). When the analyses were stratified for the individual phenotypes (axSpA, pSpA and PsA) results were similar. Taking all the possible combinations of PGA and SJC into account for the comparison between active/inactive disease, a better performance was consistently observed for the composite scores with joint counts, i.e. DAPSA, DAS28 and DAS44 (Table 3).
Conclusion: In our construct validity analysis, all disease activity instruments assessing peripheral arthritis had a good performance reflected in the correlations with external constructs and the known-groups discrimination. In the entire population, the highest discriminatory capacity to distinguish between “active and inactive disease” was observed for composite scores including joints scores, like DAPSA and DAS28.
To cite this abstract in AMA style:
Capelusnik D, Molto A, López Medina C, van der Heijde D, Landewé R, Dougados M, Sieper J, Ramiro S. Evaluation of Instruments Assessing Peripheral Arthritis in Spondyloarthritis: An Analysis of the ASAS-perSpA Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/evaluation-of-instruments-assessing-peripheral-arthritis-in-spondyloarthritis-an-analysis-of-the-asas-perspa-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-instruments-assessing-peripheral-arthritis-in-spondyloarthritis-an-analysis-of-the-asas-perspa-study/